Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism
- PMID: 23209793
- PMCID: PMC3510178
- DOI: 10.1371/journal.pone.0050629
Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism
Abstract
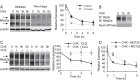
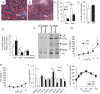
The metabolic adaptations to fasting in the liver are largely controlled by the nuclear hormone receptor peroxisome proliferator-activated receptor alpha (PPARα), where PPARα upregulates genes encoding the biochemical pathway for β-oxidation of fatty acids and ketogenesis. As part of an effort to identify and characterize nutritionally regulated genes that play physiological roles in the adaptation to fasting, we identified Major facilitator superfamily domain-containing protein 2a (Mfsd2a) as a fasting-induced gene regulated by both PPARα and glucagon signaling in the liver. MFSD2A is a cell-surface protein homologous to bacterial sodium-melibiose transporters. Hepatic expression and turnover of MFSD2A is acutely regulated by fasting/refeeding, but expression in the brain is constitutive. Relative to wildtype mice, gene-targeted Mfsd2a knockout mice are smaller, leaner, and have decreased serum, liver and brown adipose triglycerides. Mfsd2a knockout mice have normal liver lipid metabolism but increased whole body energy expenditure, likely due to increased β-oxidation in brown adipose tissue and significantly increased voluntary movement, but surprisingly exhibited a form of ataxia. Together, these results indicate that MFSD2A is a nutritionally regulated gene that plays myriad roles in body growth and development, motor function, and lipid metabolism. Moreover, these data suggest that the ligand(s) that are transported by MFSD2A play important roles in these physiological processes and await future identification.
Conflict of interest statement
Figures








References
-
- Lazo M, Clark JM (2008) The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 28: 339–350. - PubMed
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241. - PubMed
-
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, et al. (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113: 898–918. - PubMed
-
- Bonow RO, Eckel RH (2003) Diet, obesity, and cardiovascular risk. N Engl J Med 348: 2057–2058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

