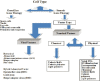
Viral and nonviral delivery systems for gene delivery
- PMID: 23210086
- PMCID: PMC3507026
- DOI: 10.4103/2277-9175.98152
Viral and nonviral delivery systems for gene delivery
Abstract
Gene therapy is the process of introducing foreign genomic materials into host cells to elicit a therapeutic benefit. Although initially the main focus of gene therapy was on special genetic disorders, now diverse diseases with different patterns of inheritance and acquired diseases are targets of gene therapy. There are 2 major categories of gene therapy, including germline gene therapy and somatic gene therapy. Although germline gene therapy may have great potential, because it is currently ethically forbidden, it cannot be used; however, to date human gene therapy has been limited to somatic cells. Although numerous viral and nonviral gene delivery systems have been developed in the last 3 decades, no delivery system has been designed that can be applied in gene therapy of all kinds of cell types in vitro and in vivo with no limitation and side effects. In this review we explain about the history of gene therapy, all types of gene delivery systems for germline (nuclei, egg cells, embryonic stem cells, pronuclear, microinjection, sperm cells) and somatic cells by viral [retroviral, adenoviral, adeno association, helper-dependent adenoviral systems, hybrid adenoviral systems, herpes simplex, pox virus, lentivirus, Epstein-Barr virus)] and nonviral systems (physical: Naked DNA, DNA bombardant, electroporation, hydrodynamic, ultrasound, magnetofection) and (chemical: Cationic lipids, different cationic polymers, lipid polymers). In addition to the above-mentioned, advantages, disadvantages, and practical use of each system are discussed.
Keywords: Chemical delivery; gene therapy; non viral delivery systems; physical delivery; viral delivery systems.
Conflict of interest statement
References
-
- Katare DP, Aeri V. Progress in gene therapy: A review. I.J.T.P.R. 2010;1:33.
-
- Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. Gene transfer into humans immunotherapy of patients with advanced melanoma, using tumor- infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. - PubMed
-
- Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after 4 years. Science. 1995;270:475–80. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources