A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran: application with clarithromycin
- PMID: 23210726
- PMCID: PMC3703233
- DOI: 10.1111/bcp.12055
A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran: application with clarithromycin
Abstract
Aim: The aim of this study was to develop a PK/PD model to assess drug-drug interactions between dabigatran and P-gp modulators, using the example of clarithromycin, a strong inhibitor of P-gp.
Methods: Ten healthy male volunteers were randomized to receive in the first treatment period a single 300 mg dose of dabigatran etexilate (DE) and in the second treatment period 500 mg clarithromycin twice daily during 3 days and then 300 mg DE plus 500 mg clarithromycin on the fourth day, or the same treatments in the reverse sequence. Dabigatran plasma concentration and ecarin clotting time (ECT) were measured on 11 blood samples. Models were built using a non-linear mixed effect modelling approach.
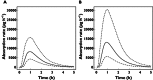
Results: The best PK model was based on an inverse Gaussian absorption process with two compartments. The relationship between dabigatran concentration and ECT was implemented as a linear function. No continuous covariate was associated with a significant decrease in the objective function. The concomitant administration of clarithromycin induced a significant change only in DE bioavailability, which increased from 6.5% to 10.1% in the presence of clarithromycin. Clarithromycin increased peak concentration and AUC by 60.2% and 49.1% respectively.
Conclusion: The model proposed effectively describes the complex PK of dabigatran and takes into account drug-drug interactions with P-gp activity modulators, such as clarithromycin.
© 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures


Similar articles
-
Switching from enoxaparin to dabigatran etexilate: pharmacokinetics, pharmacodynamics, and safety profile.Eur J Clin Pharmacol. 2012 May;68(5):607-16. doi: 10.1007/s00228-011-1205-2. Epub 2012 Jan 18. Eur J Clin Pharmacol. 2012. PMID: 22252796 Free PMC article. Clinical Trial.
-
Physiologically based pharmacokinetic modelling and in vivo [I]/K(i) accurately predict P-glycoprotein-mediated drug-drug interactions with dabigatran etexilate.Br J Pharmacol. 2014 Feb;171(4):1043-53. doi: 10.1111/bph.12533. Br J Pharmacol. 2014. PMID: 24283665 Free PMC article.
-
Oral bioavailability of dabigatran etexilate (Pradaxa(®) ) after co-medication with verapamil in healthy subjects.Br J Clin Pharmacol. 2013 Apr;75(4):1053-62. doi: 10.1111/j.1365-2125.2012.04453.x. Br J Clin Pharmacol. 2013. PMID: 22946890 Free PMC article. Clinical Trial.
-
Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate.Clin Pharmacokinet. 2008;47(5):285-95. doi: 10.2165/00003088-200847050-00001. Clin Pharmacokinet. 2008. PMID: 18399711 Review.
-
The pharmacology and therapeutic use of dabigatran etexilate.J Clin Pharmacol. 2013 Jan;53(1):1-13. doi: 10.1177/0091270011432169. Epub 2013 Jan 24. J Clin Pharmacol. 2013. PMID: 23400738 Review.
Cited by
-
Risk of Hospitalization With Hemorrhage Among Older Adults Taking Clarithromycin vs Azithromycin and Direct Oral Anticoagulants.JAMA Intern Med. 2020 Aug 1;180(8):1052-1060. doi: 10.1001/jamainternmed.2020.1835. JAMA Intern Med. 2020. PMID: 32511684 Free PMC article.
-
Physiologically-based pharmacokinetics modeling to investigate formulation factors influencing the generic substitution of dabigatran etexilate.CPT Pharmacometrics Syst Pharmacol. 2021 Mar;10(3):199-210. doi: 10.1002/psp4.12589. Epub 2021 Feb 10. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33449439 Free PMC article.
-
Binding Site Interactions of Modulators of Breast Cancer Resistance Protein, Multidrug Resistance-Associated Protein 2, and P-Glycoprotein Activity.Mol Pharm. 2020 Jul 6;17(7):2398-2410. doi: 10.1021/acs.molpharmaceut.0c00155. Epub 2020 Jun 18. Mol Pharm. 2020. PMID: 32496785 Free PMC article.
-
Association Between Use of Non-Vitamin K Oral Anticoagulants With and Without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation.JAMA. 2017 Oct 3;318(13):1250-1259. doi: 10.1001/jama.2017.13883. JAMA. 2017. PMID: 28973247 Free PMC article.
-
Drug-Drug Interaction Surveillance Study: Comparing Self-Controlled Designs in Five Empirical Examples in Real-World Data.Clin Pharmacol Ther. 2021 May;109(5):1353-1360. doi: 10.1002/cpt.2119. Epub 2020 Dec 17. Clin Pharmacol Ther. 2021. PMID: 33245789 Free PMC article.
References
-
- Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo M-L, Ahnfelt L, Piovela F, Stangier J, Lebo KA, Reilly P. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–111. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. - PubMed
-
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–399. - PubMed
-
- Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stähle H, Rathgen K, Svärd R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–563. - PubMed
-
- CHMP. Pradaxa, INN- dabigatran etexilate CHMP assessment report for radaxa. EMEA, editor. 2008 Apr 10; 1–36. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses... (last accessed 30 September 2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

