X-ray-computed tomography contrast agents
- PMID: 23210836
- PMCID: PMC3878741
- DOI: 10.1021/cr200358s
X-ray-computed tomography contrast agents
Conflict of interest statement
The authors have no conflict of interest.
Figures
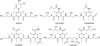
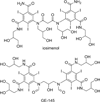
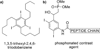
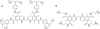


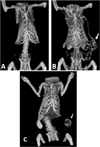

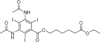


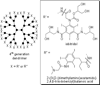


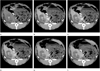
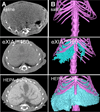




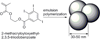

References
-
- Webb WR, Brant W, Major N. Fundamentals of body CT. 3rd ed. Philadelphia, PA: Saunders Elsevier; 2005.
- Kalender WA. Phys. Med. Biol. 2006;51:R29. - PubMed
- Buzug TM. Computed Tomography: From Photon Statistics to Modern Cone-Beam CT. 1st ed. Berlin, Germany: Springer; 2010.
- Badea CT, Drangova M, Holdsworth DW, Johnson GA. Phys. Med. Biol. 2008;53:R319. - PMC - PubMed
- Hielscher AH. Curr. Opin. Biotechnol. 2005;16:79. - PubMed
- Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Methods. 2010;50:2. - PubMed
-
- Tabulation and Graphical Summary of 2000 Survey of Computed Tomography. Conference of Radiation Control Program Directors Inc.; Frankfort, KY. 2007.
-
- Lindsten J, editor. Nobel Lectures: Physiology or Medicine 1971–1980. Vol. 1. Singapore: World Scientific Publishing Co.; 1992.
-
- Kalender WA. Computed Tomography: Fundamentals, System Technology, Image Quality, Applications. 3rd ed. Munich, Germany: Publicis; 2011.
- Romans LE. Computed Tomography for Technologists: A Comprehensive Text. 1st ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010.
- Seeram E. Computed Tomography: Physical Principles, Clinical Applications, and Quality Control. 3rd ed. St. Louis, MO: Saunders Elsevier; 2009.
- Hsieh J. Computed Tomography: Principles, Design, Artifacts, and Recent Advances. 2nd ed. Bellingham, WA: SPIE Publications; 2009.
- Figueiredo G, Brockmann C, Boll H, Heilmann M, Schambach SJ, Fiebig T, Kramer M, Groden C, Brockmann MA. Clin. Neuroradiol. 2012;22:21. - PubMed
- Figueiredo G, Boll H, Kramer M, Groden C, Brockmann MA. Am. J. Neuroradiol. 2012;33 In Press. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

