Calculation of vibrational shifts of nitrile probes in the active site of ketosteroid isomerase upon ligand binding
- PMID: 23210919
- PMCID: PMC3547160
- DOI: 10.1021/ja3084384
Calculation of vibrational shifts of nitrile probes in the active site of ketosteroid isomerase upon ligand binding
Abstract
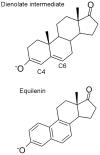
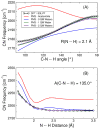
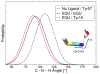
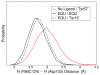
The vibrational Stark effect provides insight into the roles of hydrogen bonding, electrostatics, and conformational motions in enzyme catalysis. In a recent application of this approach to the enzyme ketosteroid isomerase (KSI), thiocyanate probes were introduced in site-specific positions throughout the active site. This paper implements a quantum mechanical/molecular mechanical (QM/MM) approach for calculating the vibrational shifts of nitrile (CN) probes in proteins. This methodology is shown to reproduce the experimentally measured vibrational shifts upon binding of the intermediate analogue equilinen to KSI for two different nitrile probe positions. Analysis of the molecular dynamics simulations provides atomistic insight into the roles that key residues play in determining the electrostatic environment and hydrogen-bonding interactions experienced by the nitrile probe. For the M116C-CN probe, equilinen binding reorients an active-site water molecule that is directly hydrogen-bonded to the nitrile probe, resulting in a more linear C≡N--H angle and increasing the CN frequency upon binding. For the F86C-CN probe, equilinen binding orients the Asp103 residue, decreasing the hydrogen-bonding distance between the Asp103 backbone and the nitrile probe and slightly increasing the CN frequency. This QM/MM methodology is applicable to a wide range of biological systems and has the potential to assist in the elucidation of the fundamental principles underlying enzyme catalysis.
Figures




References
-
- Benkovic SJ, Hammes-Schiffer S. Science. 2003;301:1196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

