Roles of Polo-like kinase 3 in suppressing tumor angiogenesis
- PMID: 23210979
- PMCID: PMC3506990
- DOI: 10.1186/2162-3619-1-5
Roles of Polo-like kinase 3 in suppressing tumor angiogenesis
Abstract
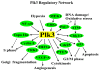
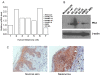
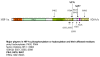
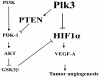
Angiogenesis is essential for promoting growth and metastasis of solid tumors by ensuring blood supply to the tumor mass. Targeting angiogenesis is therefore an attractive approach to therapeutic intervention of cancer. Tumor angiogenesis is a process that is controlled by a complex network of molecular components including sensors, signaling transducers, and effectors, leading to cellular responses under hypoxic conditions. Positioned at the center of this network are the hypoxia-inducible factors (HIFs). HIF-1 is a major transcription factor that consists of two subunits, HIF-1α and HIF-1β. It mediates transcription of a spectrum of gene targets whose products are essential for mounting hypoxic responses. HIF-1α protein level is very low in the normoxic condition but is rapidly elevated under hypoxia. This dramatic change in the cellular HIF-1α level is primarily regulated through the proteosome-mediated degradation process. In the past few years, scientific progress has clearly demonstrated that HIF-1α phosphorylation is mediated by several families of protein kinases including GSK3β and ERKs both of which play crucial roles in the regulation of HIF-1α stability. Recent research progress has identified that Polo-like kinase 3 (Plk3) phosphorylates HIF-1α at two previously unidentified serine residues and that the Plk3-mediated phosphorylation of these residues results in destabilization of HIF-1α. Plk3 has also recently been found to phosphorylate and stabilize PTEN phosphatase, a known regulator of HIF-1α and tumor angiogenesis. Given the success of targeting protein kinases and tumor angiogenesis in anti-cancer therapies, Plk3 could be a potential molecular target for the development of novel and effective therapeutic agents for cancer treatment.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

