AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator
- PMID: 23211364
- PMCID: PMC3558823
- DOI: 10.1124/jpet.112.197483
AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator
Abstract
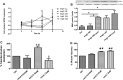
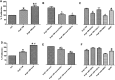
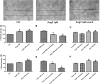
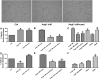
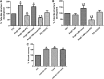
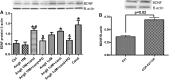
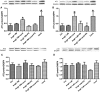
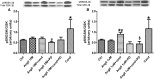
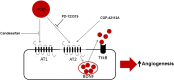
Candesartan is an angiotensin II type 1 receptor blocker (ARB) that has been to shown to limit ischemic stroke and improve stroke outcome. In experimental stroke, candesartan induces a proangiogenic effect that is partly attributable to vascular endothelial growth factor. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family that has been reported to have angiogenic effects and play an important role in recovery after stroke. The purpose of this investigation was to determine the role of BDNF in the proangiogenic effect of candesartan in the brain under hypertensive conditions. Accordingly, spontaneously hypertensive rats were treated with candesartan, and brain tissue samples were collected for quantification of BDNF expression. In addition, human cerebromicrovascular endothelial cells were treated with either low-dose (1 ƒM) or high-dose (1 µM) angiotensin II alone or in combination with candesartan (0.16 µM) to assess the effect of candesartan treatment and BDNF involvement in the behavior of endothelial cells. Candesartan significantly increased the expression of BDNF in the SHR (P < 0.05). In addition, candesartan reversed the antiangiogenic effect of the 1-µM dose of AngII (P = 0.0001). The observed effects of candesartan were ablated by neutralizing the effects of BDNF. Treatment with the AT2 antagonist PD-123319 significantly reduced tube-like formation in endothelial cells. AT2 stimulation induced the BDNF expression and migration (P < 0.05). In conclusion, candesartan exerts a proangiogenic effect on brain microvascular endothelial cells treated with angiotensin II. This response is attributable to increased BDNF expression and is mediated through stimulation of the AT2 receptor.
Figures

References
-
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group. (2002) Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359:995–1002. - PubMed
-
- Chan SH, Wu CW, Chang AY, Hsu KS, Chan JY. (2010) Transcriptional upregulation of brain-derived neurotrophic factor in rostral ventrolateral medulla by angiotensin II: significance in superoxide homeostasis and neural regulation of arterial pressure. Circ Res 107:1127–1139 - PubMed
-
- De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, Rubattu S, Volpe M. (1999) Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens 17:1873–1877 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

