β-catenin regulates GnRH-induced FSHβ gene expression
- PMID: 23211523
- PMCID: PMC3683805
- DOI: 10.1210/me.2012-1310
β-catenin regulates GnRH-induced FSHβ gene expression
Abstract
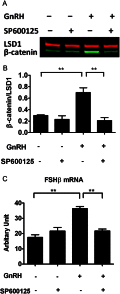
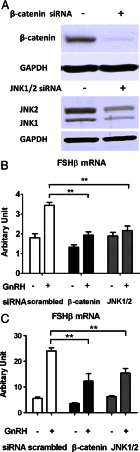
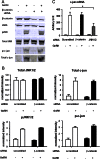
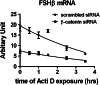
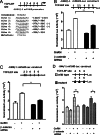
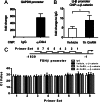
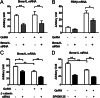
The regulation of gonadotropin synthesis by GnRH plays an essential role in the neuroendocrine control of reproduction. The known signaling mechanisms involved in gonadotropin synthesis have been expanding. For example, involvement of β-catenin in LHβ induction by GnRH has been discovered. We examined the role of β-catenin in FSHβ gene expression in LβT2 gonadotrope cells. GnRH caused a sustained increase in nuclear β-catenin levels, which was significantly reduced by c-Jun N-terminal kinase (JNK) inhibition. Small interfering RNA-mediated knockdown of β-catenin mRNA demonstrated that induction of FSHβ mRNA by GnRH depended on β-catenin and that regulation of FSHβ by β-catenin occurred independently of the JNK-c-jun pathway. β-Catenin depletion had no impact on FSHβ mRNA stability. In LβT2 cells transfected with FSHβ promoter luciferase fusion constructs, GnRH responsiveness was conferred by the proximal promoter (-944/-1) and was markedly decreased by β-catenin knockdown. However, none of the T-cell factor/lymphoid enhancer factor binding sites in that region were required for promoter activation by GnRH. Chromatin immunoprecipitation further corroborated the absence of direct interaction between β-catenin and the 1.8-kb FSHβ promoter. To elucidate the mechanism for the β-catenin effect, we analyzed approximately 1 billion reads of next-generation RNA sequencing β-catenin knockdown assays and selected the nuclear cofactor breast cancer metastasis-suppressor 1-like (Brms1L) as one candidate for further study. Subsequent experiments confirmed that Brms1L mRNA expression was decreased by β-catenin knockdown as well as by JNK inhibition. Furthermore, knockdown of Brms1L significantly attenuated GnRH-induced FSHβ expression. Thus, our findings indicate that the expression of Brms1L depends on β-catenin activity and contributes to FSHβ induction by GnRH.
Figures
References
-
- Pierce JG, Parsons TF. 1981. Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495 - PubMed
-
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. 2004. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 - PubMed
-
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. 1996. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 - PubMed
-
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. 2001. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 - PubMed
-
- Thomas P, Mellon PL, Turgeon J, Waring DW. 1996. The L β T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

