Examining T cells at vaccine sites of tumor-bearing hosts provides insights to dysfunctional T-cell immunity
- PMID: 23211619
- PMCID: PMC3521867
- DOI: 10.1097/CJI.0b013e318274590e
Examining T cells at vaccine sites of tumor-bearing hosts provides insights to dysfunctional T-cell immunity
Abstract
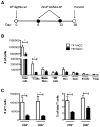
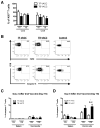
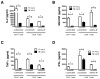
When tumor vaccines are administered as cancer immunotherapy, cellular interactions at the vaccine site are crucial to the generation of antitumor immunity. Examining interactions at the vaccine site could provide important insights to the success or failure of vaccination. Our laboratory previously showed that while administration of a cell-based vaccine to tumor-free mice leads to productive antineuroblastoma immunity, vaccination of tumor-bearing mice does not. The goal of this study was to examine immune effectors at the vaccine site to identify mechanisms responsible for the generation of ineffective antitumor immunity in tumor-bearing mice. The results of this study show that vaccine sites of tumor-bearing mice contained significantly fewer T cells than vaccine sites of tumor-free mice. Similar migration and proliferation of T cells was observed in the vaccine sites of tumor-bearing and tumor-free mice, but T cells in the sites of tumor-bearing mice were more apoptotic. T cells at the vaccine sites of both tumor-free and tumor-bearing mice had an effector-memory phenotype and expressed activation markers. Despite the activated phenotype, T cells from tumor-bearing mice elicited defective antitumor immune responses. Although T cells from vaccine sites of tumor-bearing mice were capable of producing inflammatory cytokines, the T cells from tumor-bearing mice produced lower levels of cytokines compared with T cells from the tumor-free mice. Remarkably, this defect seems to be systemic, affecting distal T cells in tumor-bearing mice. This study demonstrates that the defective vaccine-induced immune response to neuroblastoma in tumor-bearing hosts originates as a result of tumor burden, resulting in poor antitumor immunity.
Figures




Similar articles
-
Induction of immunity to neuroblastoma early after syngeneic hematopoietic stem cell transplantation using a novel mouse tumor vaccine.Biol Blood Marrow Transplant. 2007 Mar;13(3):277-92. doi: 10.1016/j.bbmt.2006.11.018. Biol Blood Marrow Transplant. 2007. PMID: 17317581 Free PMC article.
-
In vitro-activated tumor-bearing host T cells and the effectiveness of tumor vaccine immunotherapy.Ann Saudi Med. 2012 Mar-Apr;32(2):162-8. doi: 10.5144/0256-4947.2012.162. Ann Saudi Med. 2012. PMID: 22366830 Free PMC article.
-
Early expression of stem cell-associated genes within the CD8 compartment after treatment with a tumor vaccine.Cell Immunol. 2010;265(1):65-73. doi: 10.1016/j.cellimm.2010.07.004. Epub 2010 Jul 17. Cell Immunol. 2010. PMID: 20692654 Free PMC article.
-
Depletion of CD25⁺ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma.Blood. 2011 Jun 23;117(25):6952-62. doi: 10.1182/blood-2010-12-326108. Epub 2011 Apr 26. Blood. 2011. PMID: 21521781 Free PMC article.
-
Immunogene therapy.Adv Exp Med Biol. 2012;746:151-65. doi: 10.1007/978-1-4614-3146-6_12. Adv Exp Med Biol. 2012. PMID: 22639166 Review.
Cited by
-
Neuroblastoma Arginase Activity Creates an Immunosuppressive Microenvironment That Impairs Autologous and Engineered Immunity.Cancer Res. 2015 Aug 1;75(15):3043-53. doi: 10.1158/0008-5472.CAN-14-3443. Epub 2015 Jun 8. Cancer Res. 2015. PMID: 26054597 Free PMC article.
References
-
- Esiashvili N, Anderson C, Katzenstein HM. Neuroblastoma. Curr Probl Cancer. 2009;33:333–360. - PubMed
-
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. - PubMed
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

