SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism
- PMID: 23211744
- PMCID: PMC3553380
- DOI: 10.1038/emboj.2012.310
SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism
Abstract
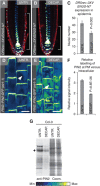
The distribution of the phytohormone auxin regulates many aspects of plant development including growth response to gravity. Gravitropic root curvature involves coordinated and asymmetric cell elongation between the lower and upper side of the root, mediated by differential cellular auxin levels. The asymmetry in the auxin distribution is established and maintained by a spatio-temporal regulation of the PIN-FORMED (PIN) auxin transporter activity. We provide novel insights into the complex regulation of PIN abundance and activity during root gravitropism. We show that PIN2 turnover is differentially regulated on the upper and lower side of gravistimulated roots by distinct but partially overlapping auxin feedback mechanisms. In addition to regulating transcription and clathrin-mediated internalization, auxin also controls PIN abundance at the plasma membrane by promoting their vacuolar targeting and degradation. This effect of elevated auxin levels requires the activity of SKP-Cullin-F-box(TIR1/AFB) (SCF(TIR1/AFB))-dependent pathway. Importantly, also suboptimal auxin levels mediate PIN degradation utilizing the same signalling pathway. These feedback mechanisms are functionally important during gravitropic response and ensure fine-tuning of auxin fluxes for maintaining as well as terminating asymmetric growth.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256 - PubMed
-
- Badescu GO, Napier RM (2006) Receptors for auxin: will it all end in TIRs? Trends Plant Sci 11: 217–223 - PubMed
-
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Péret B, Oliva M, Swarup R, Sairanen I, Parry G, Ljung K, Beeckman T, Garibaldi JM, Estelle M, Owen MR, Vissenberg K et al. (2012) Root gravitropism is regulated by a transient latera auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA 109: 4668–4673 - PMC - PubMed
-
- Barbez E, Kubeš M, Rolčík J, Béziat C, Pěnčík A, Wang B, Rosquete MR, Zhu J, Dobrev PI, Lee Y, Zažímalovà E, Petrášek J, Geisler M, Friml J, Kleine-Vehn J (2012) A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485: 119–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

