Non-destructive estimation of root pressure using sap flow, stem diameter measurements and mechanistic modelling
- PMID: 23211757
- PMCID: PMC3555520
- DOI: 10.1093/aob/mcs249
Non-destructive estimation of root pressure using sap flow, stem diameter measurements and mechanistic modelling
Abstract
Background: Upward water movement in plants via the xylem is generally attributed to the cohesion-tension theory, as a response to transpiration. Under certain environmental conditions, root pressure can also contribute to upward xylem water flow. Although the occurrence of root pressure is widely recognized, ambiguity exists about the exact mechanism behind root pressure, the main influencing factors and the consequences of root pressure. In horticultural crops, such as tomato (Solanum lycopersicum), root pressure is thought to cause cells to burst, and to have an important impact on the marketable yield. Despite the challenges of root pressure research, progress in this area is limited, probably because of difficulties with direct measurement of root pressure, prompting the need for indirect and non-destructive measurement techniques.
Methods: A new approach to allow non-destructive and non-invasive estimation of root pressure is presented, using continuous measurements of sap flow and stem diameter variation in tomato combined with a mechanistic flow and storage model, based on cohesion-tension principles.
Key results: Transpiration-driven sap flow rates are typically inversely related to stem diameter changes; however, this inverse relationship was no longer valid under conditions of low transpiration. This decoupling between sap flow rates and stem diameter variations was mathematically related to root pressure.
Conclusions: Root pressure can be estimated in a non-destructive, repeatable manner, using only external plant sensors and a mechanistic model.
Figures

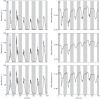
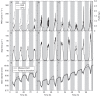
 ) of plants 1, 2 and 3 (A, B, C, respectively) and stem diameter (D) of plants 1, 2 and 3 (D, E, F, respectively) for a period of 6 d starting on 17 September 2010 at midnight. The shaded bands indicate night-time.
) of plants 1, 2 and 3 (A, B, C, respectively) and stem diameter (D) of plants 1, 2 and 3 (D, E, F, respectively) for a period of 6 d starting on 17 September 2010 at midnight. The shaded bands indicate night-time.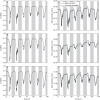



References
-
- Angeles G, Bond B, Boyer JS, et al. The cohesion–tension theory. New Phytologist. 2004;163:451–452. - PubMed
-
- van Bavel MG, van Bavel CHM. Dynagage installation and operational manual. Houston, TX: Dynamax Inc; 1990.
-
- Burquez A. Leaf thickness and water deficit in plants – a tool for field studies. Journal of Experimental Botany. 1987;38:109–114.
-
- Canny MJ. A new theory for the ascent of sap: cohesion supported by tissue pressure. Annals of Botany. 1995;75:343–357.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

