A physiological role for connective tissue growth factor in early wound healing
- PMID: 23212098
- PMCID: PMC3720136
- DOI: 10.1038/labinvest.2012.162
A physiological role for connective tissue growth factor in early wound healing
Abstract
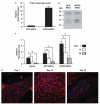
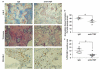
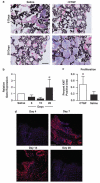
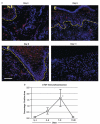
Mesenchymal stem cells (MSCs) that overexpress secreted frizzled-related protein 2 (sFRP2) exhibit an enhanced reparative phenotype. The secretomes of sFRP2-overexpressing MSCs and vector control-MSCs were compared through liquid chromatography tandem mass spectrometry. Proteomic profiling revealed that connective tissue growth factor (CTGF; CCN2) was overrepresented in the conditioned media of sFRP2-overexpressing MSCs and MSC-derived CTGF could thus be an important paracrine effector. Subcutaneously implanted, MSC-loaded polyvinyl alcohol (PVA) sponges and stented excisional wounds were used as wound models to study the dynamics of CTGF expression. Granulation tissue generated within the sponges and full-thickness skin wounds showed transient upregulation of CTGF expression by MSCs and fibroblasts, implying a role for this molecule in early tissue repair. Although collagen and COL1A2 mRNA were not increased when recombinant CTGF was administered to sponges during the early phase (day 1-6) of tissue repair, prolonged administration (>15 days) of exogenous CTGF into PVA sponges resulted in fibroblast proliferation and increased deposition of collagen within the experimental granulation tissue. In support of its physiological role, CTGF immunoinhibition during early repair (days 0-7) reduced the quantity, organizational quality and vascularity of experimental granulation tissue in the sponge model. However, CTGF haploinsufficiency was not enough to reduce collagen deposition in excisional wounds. Similar to acute murine wound models, CTGF was transiently present in the early phase of human acute burn wound healing. Together, these results further support a physiological role for CTGF in wound repair and demonstrate that when CTGF expression is confined to early tissue repair, it serves a pro-reparative role. These data also further illustrate the potential of MSC-derived paracrine modulators to enhance tissue repair.
Figures


References
-
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

