Prospects for treatment of latent HIV
- PMID: 23212106
- PMCID: PMC3942883
- DOI: 10.1038/clpt.2012.202
Prospects for treatment of latent HIV
Erratum in
- Clin Pharmacol Ther. 2013 Apr;93(4):366
Abstract
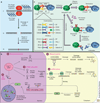
Recent advances in antiretroviral therapy (ART) have drastically improved the quality of life for people with HIV infection. However, owing to the persistence of latent HIV in the presence of therapy, patients must remain on therapy indefinitely. Currently, the solution to the HIV pandemic rests on the prevention of new infections and many decades of ART for the steadily expanding number of people infected worldwide. ART is costly, requires ongoing medical care, and can have side effects, thereby preventing its universal availability. Therefore, to escape the ironic burdens of therapy, efforts have begun to develop treatments for latent HIV infection. Current approaches propose either complete eradication of infection or induction of a state of stringent control over viral replication without ART. This review will discuss these strategies in detail and their potential for clinical development.
Conflict of interest statement
The other authors declared no conflict of interest.
Figures


Comment in
-
HIV cure: knocking on the door.Clin Pharmacol Ther. 2013 May;93(5):382-4. doi: 10.1038/clpt.2013.22. Clin Pharmacol Ther. 2013. PMID: 23598454
References
-
- WHO, UNICEF, and UNAIDS. Progress Report 2011: Global HIV/AIDS Response. 2011 < http://www.who.int/hiv/pub/progress_report2011/en/index.html>.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

