Functional regulation of P-glycoprotein at the blood-brain barrier in proton-coupled folate transporter (PCFT) mutant mice
- PMID: 23212123
- PMCID: PMC3574287
- DOI: 10.1096/fj.12-218495
Functional regulation of P-glycoprotein at the blood-brain barrier in proton-coupled folate transporter (PCFT) mutant mice
Abstract
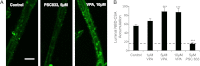
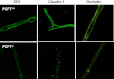
Folate deficiency has been associated with many adverse clinical manifestations. The blood-brain barrier (BBB), formed by brain capillary endothelial cells, protects the brain from exposure to neurotoxicants. The function of BBB is modulated by multiple ABC transporters, particularly P-glycoprotein. A proton-coupled folate transporter (PCFT)-deficient mouse has been previously described as a model for systemic folate deficiency. Herein, we demonstrate that exposing mouse brain capillaries to the antiepileptic drug, valproic acid (VPA; 5 μM), significantly increased P-glycoprotein transport function in the wild-type animals. A ligand to the aryl hydrocarbon receptor, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), produced a similar induction of P-glycoprotein, which tightened the BBB, thereby increasing the neuroprotection. However, VPA- or TCDD-induced P-glycoprotein transport was blocked in the PCFT-nullizygous mice, indicating that multiple neuroprotective mechanisms are compromised under folate-deficient conditions. Brain capillaries from S-folinic acid (SFA; 40 mg/kg)-treated PCFT-nullizygous mice exhibited increased P-glycoprotein transport following VPA exposure. This suggests that SFA supplementation restored the normal BBB function. In addition, we show that tight-junction proteins are disintegrated in the PCFT mutant mice. Taken together, these findings strongly suggest that folate deficiency disrupts the BBB function by targeting the transporter and tight junctions, which may contribute to the development of neurological disorders.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

