Synaptic tagging and capture in the living rat
- PMID: 23212375
- PMCID: PMC3705498
- DOI: 10.1038/ncomms2250
Synaptic tagging and capture in the living rat
Abstract
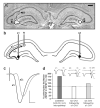
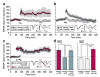
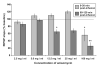
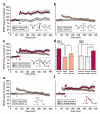
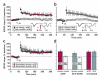
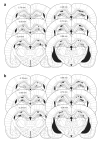
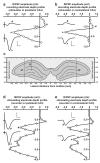
In isolated hippocampal slices, decaying long-term potentiation can be stabilized and converted to late long-term potentiation lasting many hours, by prior or subsequent strong high-frequency tetanization of an independent input to a common population of neurons-a phenomenon known as 'synaptic tagging and capture'. Here we show that the same phenomenon occurs in the intact rat. Late long-term potentiation can be induced in CA1 during the inhibition of protein synthesis if an independent input is strongly tetanized beforehand. Conversely, declining early long-term potentiation induced by weak tetanization can be converted into lasting late long-term potentiation by subsequent strong tetanization of a separate input. These findings indicate that synaptic tagging and capture is not limited to in vitro preparations; the past and future activity of neurons has a critical role in determining the persistence of synaptic changes in the living animal, thus providing a bridge between cellular studies of protein synthesis-dependent synaptic potentiation and behavioural studies of memory persistence.
Figures
References
-
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. - PubMed
-
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. - PubMed
-
- Hubener M, Bonhoeffer T. Searching for engrams. Neuron. 2010;67:363–371. - PubMed
-
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol. Bull. 2006;210:174–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

