Identification and characterization of a human mitochondrial NAD kinase
- PMID: 23212377
- PMCID: PMC3535332
- DOI: 10.1038/ncomms2262
Identification and characterization of a human mitochondrial NAD kinase
Abstract
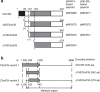
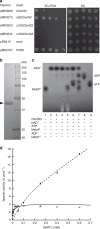
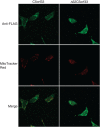
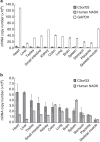
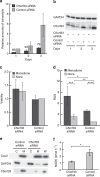
NAD kinase is the sole NADP(+) biosynthetic enzyme. Despite the great significance of NADP(+), to date no mitochondrial NAD kinase has been identified in human, and the source of human mitochondrial NADP(+) remains elusive. Here we present evidence demonstrating that a human protein of unknown function, C5orf33, is a human mitochondrial NAD kinase; this protein likely represents the missing source of human mitochondrial NADP(+). The C5orf33 protein exhibits NAD kinase activity, utilizing ATP or inorganic polyphosphate, and is localized in the mitochondria of human HEK293A cells. C5orf33 mRNA is more abundant than human cytosolic NAD kinase mRNA in almost all tissues examined. We further show by database searches that some animals and protists carry C5orf33 homologues as their sole NADP(+) biosynthetic enzyme, whereas plants and fungi possess no C5orf33 homologue. These observations provide insights into eukaryotic NADP(+) biosynthesis, which has pivotal roles in cells and organelles.
Figures
References
-
- Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur. J. Biochem. 267, 1550–1564 (2000). - PubMed
-
- Yamasaki M. et al.. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J. Biol. Chem. 279, 7234–7240 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

