In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells
- PMID: 23212524
- PMCID: PMC3701251
- DOI: 10.1182/blood-2012-06-434407
In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells
Abstract
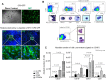
Lineage-restricted cells can be reprogrammed to a pluripotent state known as induced pluripotent stem (iPS) cells through overexpression of 4 transcription factors. iPS cells are similar to human embryonic stem (hES) cells and have the same ability to generate all the cells of the human body, including blood cells. However, this process is extremely inefficient and to date has been unsuccessful at differentiating iPS into hematopoietic stem cells (HSCs). We hypothesized that iPS cells, injected into NOD.Cg-Prkdc(scid) Il2rg(tm1Wjl)/SzJ immunocompromised (NSG) mice could give rise to hematopoietic stem/progenitor cells (HSPCs) during teratoma formation. Here, we report a novel in vivo system in which human iPS cells differentiate within teratomas to derive functional myeloid and lymphoid cells. Similarly, HSPCs can be isolated from teratoma parenchyma and reconstitute a human immune system when transplanted into immunodeficient mice. Our data provide evidence that in vivo generation of patient customized cells is feasible, providing materials that could be useful for transplantation, human antibody generation, and drug screening applications.
Figures




Comment in
-
Tu-mor(e) blood cells from human pluripotent stem cells.Blood. 2013 Feb 21;121(8):1245-6. doi: 10.1182/blood-2013-01-472563. Blood. 2013. PMID: 23429981
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
-
- Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. - PubMed
-
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. - PubMed
-
- Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. - PubMed

