Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice
- PMID: 23212557
- PMCID: PMC6408949
- DOI: 10.1007/s00395-012-0315-z
Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice
Abstract
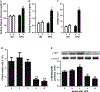
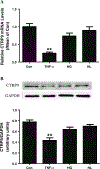
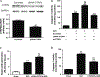
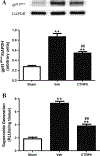
Recently identified as adiponectin (APN) paralogs, C1q/TNF-related proteins (CTRPs) share similar metabolic regulatory functions as APN. The current study determined cardiac expression of CTRPs, their potential cardioprotective function, and investigated whether and how diabetes may regulate cardiac CTRP expression. Several CTRPs are expressed in the heart at levels significantly greater than APN. Most notably, cardiac expression of CTRP9, the closest paralog of APN, exceeds APN by >100-fold. Cardiac CTRP9 expression was significantly reduced in high-fat diet-induced diabetic mice. In H9c2 cells, tumor necrosis factor-alpha (TNF-α) strongly inhibited CTRP9 expression (>60%), and significantly reduced peroxisome proliferator activated receptor-gamma (PPARγ), a known transcription factor promoting adiponectin expression. The inhibitory effect of TNF-α on PPARγ and CTRP9 was reversed by Tiron or rosiglitazone. CTRP9 knockdown significantly enhanced, whereas CTRP9 overexpression significantly attenuated simulated ischemia/reperfusion injury in H9c2 cells. In vivo CTRP9 administration to diabetic mice significantly attenuated NADPH oxidase expression and superoxide generation, reduced infarct size, and improved cardiac function. To the best of our knowledge, this is the first study providing evidence that downregulation of CTRP9, an abundantly expressed and novel cell survival molecule in the heart, by TNF-α-initiated oxidative PPARγ suppression contributes to exacerbated diabetic cardiac injury. Preservation of CTRP9 expression or augmentation of CTRP9-initiated signaling mechanisms may be the potential avenues for ameliorating ischemic diabetic cardiac injury.
Figures





Similar articles
-
C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway.Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2616-23. doi: 10.1161/ATVBAHA.111.231050. Arterioscler Thromb Vasc Biol. 2011. PMID: 21836066 Free PMC article.
-
C1q-TNF-related protein-9, a novel cardioprotetcive cardiokine, requires proteolytic cleavage to generate a biologically active globular domain isoform.Am J Physiol Endocrinol Metab. 2015 May 15;308(10):E891-8. doi: 10.1152/ajpendo.00450.2014. Epub 2015 Mar 17. Am J Physiol Endocrinol Metab. 2015. PMID: 25783894 Free PMC article.
-
C1q/TNF-Related Protein 9 Protects Diabetic Rat Heart against Ischemia Reperfusion Injury: Role of Endoplasmic Reticulum Stress.Oxid Med Cell Longev. 2016;2016:1902025. doi: 10.1155/2016/1902025. Epub 2016 Oct 4. Oxid Med Cell Longev. 2016. PMID: 27795806 Free PMC article.
-
CTRP9: An emerging potential anti-aging molecule in brain.Cell Signal. 2020 Sep;73:109694. doi: 10.1016/j.cellsig.2020.109694. Epub 2020 Jun 12. Cell Signal. 2020. PMID: 32540339 Review.
-
C1q/Tumor Necrosis Factor-Related Protein 9: Basics and Therapeutic Potentials.Front Physiol. 2022 Mar 18;13:816218. doi: 10.3389/fphys.2022.816218. eCollection 2022. Front Physiol. 2022. PMID: 35370782 Free PMC article. Review.
Cited by
-
Impact of PCSK9 on CTRP9-Induced Metabolic Effects in Adult Rat Cardiomyocytes.Front Physiol. 2021 Feb 11;12:593862. doi: 10.3389/fphys.2021.593862. eCollection 2021. Front Physiol. 2021. PMID: 33643060 Free PMC article.
-
Integrative analysis reveals novel associations between DNA methylation and the serum metabolome of adolescents with type 2 diabetes: A cross-sectional study.Front Endocrinol (Lausanne). 2022 Oct 10;13:934706. doi: 10.3389/fendo.2022.934706. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36303872 Free PMC article.
-
Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency.Exp Mol Med. 2022 Feb;54(2):103-114. doi: 10.1038/s12276-022-00728-w. Epub 2022 Feb 3. Exp Mol Med. 2022. PMID: 35115674 Free PMC article.
-
Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers.Int J Mol Sci. 2023 Jan 3;24(1):844. doi: 10.3390/ijms24010844. Int J Mol Sci. 2023. PMID: 36614282 Free PMC article. Review.
-
Complement 1q/Tumor Necrosis Factor-Related Proteins (CTRPs): Structure, Receptors and Signaling.Biomedicines. 2023 Feb 14;11(2):559. doi: 10.3390/biomedicines11020559. Biomedicines. 2023. PMID: 36831095 Free PMC article. Review.
References
-
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL (2002) Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105:1497–1502. doi:10.1161/01.CIR.0000012529.00367.0F - DOI - PubMed
-
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM (2003) Adipose-specific peroxi-some proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100:15712–15717. doi:10.1073/pnas.2536828100 - DOI - PMC - PubMed
-
- Iaconetti C, Polimeni A, Sorrentino S, Sabatino J, Pironti G, Esposito G, Curcio A, Indolfi C (2012) Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res Cardiol 107:296. doi:10.1007/s00395-012-0296-y - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

