Mitotic exit and separation of mother and daughter cells
- PMID: 23212898
- PMCID: PMC3512134
- DOI: 10.1534/genetics.112.145516
Mitotic exit and separation of mother and daughter cells
Abstract
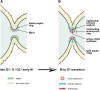
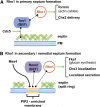
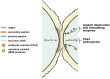
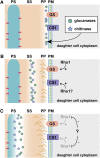
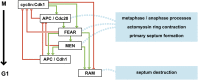
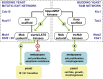
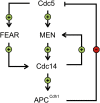
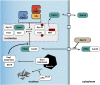
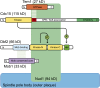
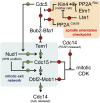
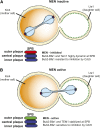
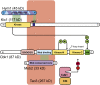
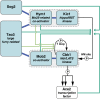
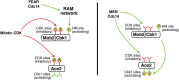
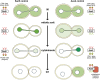
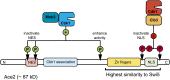
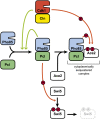
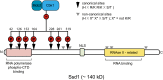
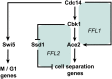
Productive cell proliferation involves efficient and accurate splitting of the dividing cell into two separate entities. This orderly process reflects coordination of diverse cytological events by regulatory systems that drive the cell from mitosis into G1. In the budding yeast Saccharomyces cerevisiae, separation of mother and daughter cells involves coordinated actomyosin ring contraction and septum synthesis, followed by septum destruction. These events occur in precise and rapid sequence once chromosomes are segregated and are linked with spindle organization and mitotic progress by intricate cell cycle control machinery. Additionally, critical paarts of the mother/daughter separation process are asymmetric, reflecting a form of fate specification that occurs in every cell division. This chapter describes central events of budding yeast cell separation, as well as the control pathways that integrate them and link them with the cell cycle.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

