Critical role of S1PR1 and integrin β4 in HGF/c-Met-mediated increases in vascular integrity
- PMID: 23212923
- PMCID: PMC3554892
- DOI: 10.1074/jbc.M112.404780
Critical role of S1PR1 and integrin β4 in HGF/c-Met-mediated increases in vascular integrity
Abstract
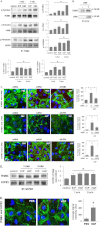
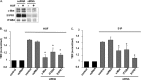
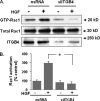
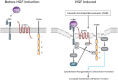
Vascular endothelial cell (EC) barrier integrity is critical to vessel homeostasis whereas barrier dysfunction is a key feature of inflammatory disorders and tumor angiogenesis. We previously reported that hepatocyte growth factor (HGF)-mediated increases in EC barrier integrity are signaled through a dynamic complex present in lipid rafts involving its receptor, c-Met. We extended these observations to confirm that S1PR1 (sphingosine 1-phosphate receptor 1) and integrin β4 (ITGB4) are essential participants in HGF-induced EC barrier enhancement. Immunoprecipitation experiments demonstrated HGF-mediated recruitment of c-Met, ITGB4 and S1PR1 to caveolin-enriched lipid rafts in human lung EC with direct interactions of c-Met with both S1PR1 and ITGB4 accompanied by c-Met-dependent S1PR1 and ITGB4 transactivation. Reduced S1PR1 expression (siRNA) attenuated both ITGB4 and Rac1 activation as well as c-Met/ITGB4 interaction and resulted in decreased transendothelial electrical resistance. Furthermore, reduced ITGB4 expression attenuated HGF-induced c-Met activation, c-Met/S1PR1 interaction, and effected decreases in S1P- and HGF-induced EC barrier enhancement. Finally, the c-Met inhibitor, XL880, suppressed HGF-induced c-Met activation as well as S1PR1 and ITGB4 transactivation. These results support a critical role for S1PR1 and ITGB4 transactivation as rate-limiting events in the transduction of HGF signals via a dynamic c-Met complex resulting in enhanced EC barrier integrity.
Figures




References
-
- Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. (2007) CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J. Biol. Chem. 282, 30643–30657 - PubMed
-
- Dudek S. M., Jacobson J. R., Chiang E. T., Birukov K. G., Wang P., Zhan X., Garcia J. G. (2004) Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J. Biol. Chem. 279, 24692–24700 - PubMed
-
- Singleton P. A., Dudek S. M., Chiang E. T., Garcia J. G. (2005) Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1PR1 receptor, PI3 kinase, Tiam1/Rac1, and α-actinin. Faseb J. 19, 1646–1656 - PubMed
-
- Singleton P. A., Dudek S. M., Ma S. F., Garcia J. G. (2006) Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J. Biol. Chem. 281, 34381–34393 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

