Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation
- PMID: 23213032
- PMCID: PMC3674713
- DOI: 10.1093/gerona/gls238
Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation
Abstract
Obesity in the elderly individuals is increasing at alarming rates and there is evidence suggesting that elderly individuals are more vulnerable to the deleterious cardiovascular effects of obesity than younger individuals. However, the specific mechanisms through which aging and obesity interact to promote the development of cardiovascular disease remain unclear. The present study was designed to test the hypothesis that aging exacerbates obesity-induced inflammation in perivascular adipose tissue, which contributes to increased vascular oxidative stress and inflammation in a paracrine manner. To test this hypothesis, we assessed changes in the secretome, reactive oxygen species production, and macrophage infiltration in periaortic adipose tissue of young (7 month old) and aged (24 month old) high-fat diet-fed obese C57BL/6 mice. High-fat diet-induced vascular reactive oxygen species generation significantly increased in aged mice, which was associated with exacerbation of endothelial dysfunction and vascular inflammation. In young animals, high-fat diet-induced obesity promoted oxidative stress in the perivascular adipose tissue, which was associated with a marked proinflammatory shift in the profile of secreted cytokines and chemokines. Aging exacerbated obesity-induced oxidative stress and inflammation and significantly increased macrophage infiltration in periaortic adipose tissue. Using cultured arteries isolated from young control mice, we found that inflammatory factors secreted from the perivascular fat tissue of obese aged mice promote significant prooxidative and proinflammatory phenotypic alterations in the vascular wall, mimicking the aging phenotype. Overall, our findings support an important role for localized perivascular adipose tissue inflammation in exacerbation of vascular oxidative stress and inflammation in aging, an effect that likely enhances the risk for development of cardiovascular diseases from obesity in the elderly individuals.
Keywords: Adiposity; Diabetes; Fat; Metabolic disease; Obesity.
Figures
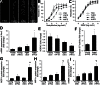
 production, in sections of aortas isolated from young and aged mice fed a HFD or standard diet (SD). For orientation purposes, overlay of the nuclear DHE signal (excitation: 490nm, emission: 525nm) and autofluorescence of elastic laminae (excitation: 490nm, emission: 525nm) is shown. Original magnification: 20×. (Panels B and C) Relaxation of aorta rings isolated from young and aged SD- or HFD-fed mice. Vasomotor responses were induced by the endothelium-dependent agent acetylcholine (B) and S-nitroso-N-acetylpenicillamine (SNAP, Panel C), an endothelium-independent vasodilator. Data are means ± SEM; *p < .05 vs respective SD-fed control (n = 5–7). (Panel D) Quantitative real-time RT-PCR data showing mRNA expression of Nox2 in the aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel E) Hydroxyl Radical Antioxidant Capacity (HORAC) in the aorta of young and aged SD- and HFD-fed mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel F) HFD-induced increases in caspase 3/7 activity in aortas of young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05. (Panels G and H) Protein expression of MCP-1 (G) and MIP-1α (H) in aortas of HFD-fed and SD-fed young and aged mice. Data are means ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young. (Panel I) mRNA expression of the senescence marker p16INK4a in aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young.
production, in sections of aortas isolated from young and aged mice fed a HFD or standard diet (SD). For orientation purposes, overlay of the nuclear DHE signal (excitation: 490nm, emission: 525nm) and autofluorescence of elastic laminae (excitation: 490nm, emission: 525nm) is shown. Original magnification: 20×. (Panels B and C) Relaxation of aorta rings isolated from young and aged SD- or HFD-fed mice. Vasomotor responses were induced by the endothelium-dependent agent acetylcholine (B) and S-nitroso-N-acetylpenicillamine (SNAP, Panel C), an endothelium-independent vasodilator. Data are means ± SEM; *p < .05 vs respective SD-fed control (n = 5–7). (Panel D) Quantitative real-time RT-PCR data showing mRNA expression of Nox2 in the aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel E) Hydroxyl Radical Antioxidant Capacity (HORAC) in the aorta of young and aged SD- and HFD-fed mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel F) HFD-induced increases in caspase 3/7 activity in aortas of young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05. (Panels G and H) Protein expression of MCP-1 (G) and MIP-1α (H) in aortas of HFD-fed and SD-fed young and aged mice. Data are means ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young. (Panel I) mRNA expression of the senescence marker p16INK4a in aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young.
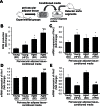
 production in sections of perivascular adipose tissue isolated from standard diet (SD)–fed and HFD-fed young and aged mice. Note that tissues derived from HFD-fed aged mice exhibit the most intense DHE fluorescence. Original magnification: 20×. (Panel B) Representative micrographs showing brown immunostaining for CD68 in sections of perivascular adipose tissues isolated from SD- and HFD-fed young and aged mice. Tissues derived from HFD-fed aged mice exhibit the greatest infiltration by CD68+ macrophages (arrows). In SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes. In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed. Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. (Panel C) Summary data for relative age- and HFD-induced changes in CD68+ macrophage content in perivascular adipose tissue. Data are mean ± SEM; *p < .05 vs control.
production in sections of perivascular adipose tissue isolated from standard diet (SD)–fed and HFD-fed young and aged mice. Note that tissues derived from HFD-fed aged mice exhibit the most intense DHE fluorescence. Original magnification: 20×. (Panel B) Representative micrographs showing brown immunostaining for CD68 in sections of perivascular adipose tissues isolated from SD- and HFD-fed young and aged mice. Tissues derived from HFD-fed aged mice exhibit the greatest infiltration by CD68+ macrophages (arrows). In SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes. In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed. Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. (Panel C) Summary data for relative age- and HFD-induced changes in CD68+ macrophage content in perivascular adipose tissue. Data are mean ± SEM; *p < .05 vs control.

References
-
- Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007;15:2855–2865 - PubMed
-
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977 - PubMed
-
- Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

