CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8
- PMID: 23213055
- PMCID: PMC3548984
- DOI: 10.1158/1078-0432.CCR-12-2153
CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8
Abstract
Purpose: Controversy exists about CYP2D6 genotype and tamoxifen efficacy.
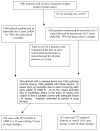
Experimental design: A matched case-control study was conducted using the Austrian Breast and Colorectal Cancer Study Group Trial 8 (ABCSG8) that randomized postmenopausal women with estrogen receptor (ER)-positive breast cancer to tamoxifen for 5 years (arm A) or tamoxifen for 2 years followed by anastrozole for 3 years (arm B). Cases had disease recurrence, contralateral breast cancer, second non-breast cancer, or died. For each case, controls were identified from the same treatment arm of similar age, surgery/radiation, and tumor-node-metastasis (TNM) stage. Genotyping was conducted for alleles associated with no (PM; *3, *4, *6), reduced (IM; *10, and *41), and extensive (EM: absence of these alleles) CYP2D6 metabolism.
Results: The common CYP2D6*4 allele was in Hardy-Weinberg equilibrium. In arm A during the first 5 years of therapy, women with two poor alleles [PM/PM: OR, 2.45; 95% confidence interval (CI), 1.05-5.73, P = 0.04] and women with one poor allele (PM/IM or PM/EM: OR, 1.67; 95% CI, 0.95-2.93; P = 0.07) had a higher likelihood of an event than women with two extensive alleles (EM/EM). In years 3 to 5 when patients remained on tamoxifen (arm A) or switched to anastrozole (arm B), PM/PM tended toward a higher likelihood of a disease event relative to EM/EM (OR, 2.40; 95% CI, 0.86-6.66; P = 0.09) among women on arm A but not among women on arm B (OR, 0.28; 95% CI, 0.03-2.30).
Conclusion: In ABCSG8, the negative effects of reduced CYP2D6 metabolism were observed only during the period of tamoxifen administration and not after switching to anastrozole.
©2012 AACR.
References
-
- Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:509–18. - PubMed
-
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 28:3784–96. - PMC - PubMed
-
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, et al. Pharmacological characterization of 4-hydroxy- N -desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast cancer research and treatment. 2004;85:151–9. - PubMed
-
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64. - PubMed
-
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases