Central nervous system Gαi2-subunit proteins maintain salt resistance via a renal nerve-dependent sympathoinhibitory pathway
- PMID: 23213191
- PMCID: PMC3562703
- DOI: 10.1161/HYPERTENSIONAHA.111.00014
Central nervous system Gαi2-subunit proteins maintain salt resistance via a renal nerve-dependent sympathoinhibitory pathway
Abstract
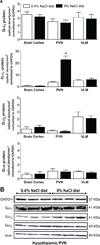
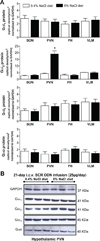
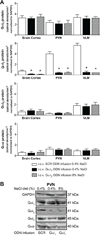
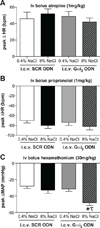
In salt-resistant phenotypes, chronic elevated dietary sodium intake evokes suppression of renal sodium-retaining mechanisms to maintain sodium homeostasis and normotension. We have recently shown that brain Gαi(2) protein pathways are required to suppress renal sympathetic nerve activity and facilitate maximal sodium excretion during acute intravenous volume expansion in Sprague-Dawley rats. Here, we studied the role of brain Gαi(2) proteins in the endogenous central neural mechanisms acting to maintain fluid and electrolyte homeostasis and normotension during a chronic elevation in dietary salt intake. Naive or bilaterally renal denervated adult male Sprague-Dawley rats were randomly assigned to receive an intracerebroventricular scrambled or Gαi(2) oligodeoxynucleotide infusion and then subjected to either a normal salt (0.4%) or high-salt (8.0%) diet for 21 days. In scrambled oligodeoxynucleotide-infused rats, salt loading, which did not alter blood pressure, evoked a site-specific increase in hypothalamic paraventricular nucleus Gαi(2) protein levels and suppression of circulating norepinephrine content and plasma renin activity. In salt-loaded rats continuously infused intracerebroventricularly with a Gαi(2) oligodeoxynucleotide, animals exhibited sodium and water retention, elevated plasma norepinephrine levels, and hypertension, despite suppression of plasma renin activity. Furthermore, in salt-loaded bilaterally renal denervated rats, Gαi(2) oligodeoxynucleotide infusion failed to evoke salt-sensitive hypertension. Therefore, in salt-resistant rats subjected to a chronic high-salt diet, brain Gαi(2) proteins are required to inhibit central sympathetic outflow to the kidneys and maintain sodium balance and normotension. In conclusion, these data demonstrate a central role of endogenous brain, likely paraventricular nucleus-specific, Gαi(2)-subunit protein-gated signal transduction pathways in maintaining a salt-resistant phenotype.
Conflict of interest statement
Figures
Similar articles
-
Gαi2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension.Hypertension. 2015 Jan;65(1):178-86. doi: 10.1161/HYPERTENSIONAHA.114.04463. Epub 2014 Oct 13. Hypertension. 2015. PMID: 25312437 Free PMC article.
-
Brain Gαi2-subunit protein-gated pathways are required to mediate the centrally evoked sympathoinhibitory mechanisms activated to maintain sodium homeostasis.J Hypertens. 2013 Apr;31(4):747-57. doi: 10.1097/HJH.0b013e32835ebd54. J Hypertens. 2013. PMID: 23391983
-
Hypothalamic Paraventricular Nucleus Gαi2 (Guanine Nucleotide-Binding Protein Alpha Inhibiting Activity Polypeptide 2) Protein-Mediated Neural Control of the Kidney and the Salt Sensitivity of Blood Pressure.Hypertension. 2020 Apr;75(4):1002-1011. doi: 10.1161/HYPERTENSIONAHA.119.13777. Epub 2020 Mar 9. Hypertension. 2020. PMID: 32148128 Free PMC article.
-
Central Gαi2 Protein Mediated Neuro-Hormonal Control of Blood Pressure and Salt Sensitivity.Front Endocrinol (Lausanne). 2022 Jun 28;13:895466. doi: 10.3389/fendo.2022.895466. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837296 Free PMC article. Review.
-
Brain Gαi 2 -subunit proteins and the prevention of salt sensitive hypertension.Front Physiol. 2015 Aug 19;6:233. doi: 10.3389/fphys.2015.00233. eCollection 2015. Front Physiol. 2015. PMID: 26347659 Free PMC article. Review.
Cited by
-
Salt sensitivity and hypertension.J Hum Hypertens. 2021 Mar;35(3):184-192. doi: 10.1038/s41371-020-00407-1. Epub 2020 Aug 29. J Hum Hypertens. 2021. PMID: 32862203 Review.
-
Sensory Afferent Renal Nerve Activated Gαi2 Subunit Proteins Mediate the Natriuretic, Sympathoinhibitory and Normotensive Responses to Peripheral Sodium Challenges.Front Physiol. 2021 Nov 30;12:771167. doi: 10.3389/fphys.2021.771167. eCollection 2021. Front Physiol. 2021. PMID: 34916958 Free PMC article.
-
Integrated renal and sympathetic mechanisms underlying the development of sex- and age-dependent hypertension and the salt sensitivity of blood pressure.Geroscience. 2024 Dec;46(6):6435-6458. doi: 10.1007/s11357-024-01266-1. Epub 2024 Jul 8. Geroscience. 2024. PMID: 38976131 Free PMC article.
-
Sympathetic regulation of NCC in norepinephrine-evoked salt-sensitive hypertension in Sprague-Dawley rats.Am J Physiol Renal Physiol. 2019 Dec 1;317(6):F1623-F1636. doi: 10.1152/ajprenal.00264.2019. Epub 2019 Oct 14. Am J Physiol Renal Physiol. 2019. PMID: 31608673 Free PMC article.
-
Sex differences in dietary sodium evoked NCC regulation and blood pressure in male and female Sprague-Dawley, Dahl salt-resistant, and Dahl salt-sensitive rats.Am J Physiol Renal Physiol. 2024 Aug 1;327(2):F277-F289. doi: 10.1152/ajprenal.00150.2023. Epub 2024 May 30. Am J Physiol Renal Physiol. 2024. PMID: 38813592 Free PMC article.
References
-
- Franco V, Oparil S. Salt Sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Med Coll Nutrit. 2006;25:247S–255S. - PubMed
-
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular disease. Physiol Rev. 2005;85:679–715. - PubMed
-
- Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension – update on novel findings. Nephrol Dial Transplant. 2007;22:992–995. - PubMed
-
- Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32:426–432. - PubMed
-
- Lohmeier TE, Hildebrandt W, Hood A. Renal nerves promote sodium excretion during long-term increases in salt-intake. Hypertension. 1999;33:487–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

