Possible role of pineal allopregnanolone in Purkinje cell survival
- PMID: 23213208
- PMCID: PMC3529028
- DOI: 10.1073/pnas.1210804109
Possible role of pineal allopregnanolone in Purkinje cell survival
Abstract
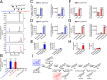
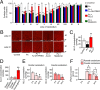
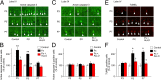
It is believed that neurosteroids are produced in the brain and other nervous systems. Here, we show that allopregnanolone (ALLO), a neurosteroid, is exceedingly produced in the pineal gland compared with the brain and that pineal ALLO acts on the Purkinje cell, a principal cerebellar neuron, to prevent apoptosis in the juvenile quail. We first demonstrated that the pineal gland is a major organ of neurosteroidogenesis. A series of experiments using molecular and biochemical techniques has further demonstrated that the pineal gland produces a variety of neurosteroids de novo from cholesterol in the juvenile quail. Importantly, ALLO was far more actively produced in the pineal gland than in the brain. Pinealectomy (Px) decreased ALLO concentration in the cerebellum and induced apoptosis of Purkinje cells, whereas administration of ALLO to Px quail chicks prevented apoptosis of Purkinje cells. We further found that Px significantly increased the number of Purkinje cells that expressed active caspase-3, a key protease in apoptotic pathway, and daily injection of ALLO to Px quail chicks decreased the number of Purkinje cells expressing active caspase-3. These results indicate that the neuroprotective effect of pineal ALLO is associated with the decrease in caspase-3 activity during the early stage of neuronal development. We thus provide evidence that the pineal gland is an important neurosteroidogenic organ and that pineal ALLO may be involved in Purkinje cell survival during development. This is an important function of the pineal gland in the formation of neuronal circuits in the developing cerebellum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Neuroprotective actions of cerebellar and pineal allopregnanolone on Purkinje cells.FASEB Bioadv. 2020 Feb 5;2(3):149-159. doi: 10.1096/fba.2019-00055. eCollection 2020 Mar. FASEB Bioadv. 2020. PMID: 32161904 Free PMC article. Review.
-
Biosynthesis and biological action of pineal allopregnanolone.Front Cell Neurosci. 2014 May 5;8:118. doi: 10.3389/fncel.2014.00118. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24834027 Free PMC article. Review.
-
Kobayashi award: Discovery of cerebellar and pineal neurosteroids and their biological actions on the growth and survival of Purkinje cells during development (review).Gen Comp Endocrinol. 2019 Dec 1;284:113051. doi: 10.1016/j.ygcen.2018.10.014. Epub 2018 Oct 16. Gen Comp Endocrinol. 2019. PMID: 30339808 Review.
-
Pineal Neurosteroids: Biosynthesis and Physiological Functions.Front Endocrinol (Lausanne). 2020 Aug 11;11:549. doi: 10.3389/fendo.2020.00549. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32849313 Free PMC article. Review.
-
Light-at-night exposure affects brain development through pineal allopregnanolone-dependent mechanisms.Elife. 2019 Sep 30;8:e45306. doi: 10.7554/eLife.45306. Elife. 2019. PMID: 31566568 Free PMC article.
Cited by
-
The impact of light during the night.Elife. 2019 Nov 12;8:e52364. doi: 10.7554/eLife.52364. Elife. 2019. PMID: 31714876 Free PMC article.
-
New biosynthesis and biological actions of avian neurosteroids.J Exp Neurosci. 2013 May 27;7:15-29. doi: 10.4137/JEN.S11148. eCollection 2013. J Exp Neurosci. 2013. PMID: 25157204 Free PMC article. Review.
-
Neuroprotective actions of cerebellar and pineal allopregnanolone on Purkinje cells.FASEB Bioadv. 2020 Feb 5;2(3):149-159. doi: 10.1096/fba.2019-00055. eCollection 2020 Mar. FASEB Bioadv. 2020. PMID: 32161904 Free PMC article. Review.
-
De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function.Int J Mol Sci. 2020 May 7;21(9):3316. doi: 10.3390/ijms21093316. Int J Mol Sci. 2020. PMID: 32392845 Free PMC article. Review.
-
Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis.Nat Commun. 2014;5:3061. doi: 10.1038/ncomms4061. Nat Commun. 2014. PMID: 24430094 Free PMC article.
References
-
- Kilic E, Hermann DM, Isenmann S, Bähr M. Effects of pinealectomy and melatonin on the retrograde degeneration of retinal ganglion cells in a novel model of intraorbital optic nerve transection in mice. J Pineal Res. 2002;32(2):106–111. - PubMed
-
- Tunç AT, et al. Neonatal pinealectomy induces Purkinje cell loss in the cerebellum of the chick: A stereological study. Brain Res. 2006;1067(1):95–102. - PubMed
-
- Baulieu EE. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. - PubMed
-
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. - PubMed
-
- Do Rego JL, et al. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30(3):259–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

