Dendritic upconverting nanoparticles enable in vivo multiphoton microscopy with low-power continuous wave sources
- PMID: 23213211
- PMCID: PMC3529031
- DOI: 10.1073/pnas.1213291110
Dendritic upconverting nanoparticles enable in vivo multiphoton microscopy with low-power continuous wave sources
Abstract
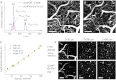
We report a group of optical imaging probes, comprising upconverting lanthanide nanoparticles (UCNPs) and polyanionic dendrimers. Dendrimers with rigid cores and multiple carboxylate groups at the periphery are able to tightly bind to surfaces of UCNPs pretreated with NOBF(4), yielding stable, water-soluble, biocompatible nanomaterials. Unlike conventional linear polymers, dendrimers adhere to UCNPs by donating only a fraction of their peripheral groups to the UCNP-surface interactions. The remaining termini make up an interface between the nanoparticle and the aqueous phase, enhancing solubility and offering multiple possibilities for subsequent modification. Using optical probes as dendrimer cores makes it possible to couple the UCNPs signal to analyte-sensitive detection via UCNP-to-chromophore excitation energy transfer (EET). As an example, we demonstrate that UCNPs modified with porphyrin-dendrimers can operate as upconverting ratiometric pH nanosensors. Dendritic UCNPs possess excellent photostability, solubility, and biocompatibility, which make them directly suitable for in vivo imaging. Polyglutamic dendritic UCNPs injected in the blood of a mouse allowed mapping of the cortical vasculature down to 400 μm under the tissue surface, thus demonstrating feasibility of in vivo high-resolution two-photon microscopy with continuous wave (CW) excitation sources. Dendrimerization as a method of solubilization of UCNPs opens up numerous possibilities for use of these unique agents in biological imaging and sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
In vivo deep-tissue microscopy with UCNP/Janus-dendrimers as imaging probes: resolution at depth and feasibility of ratiometric sensing.Nanoscale. 2020 Jan 28;12(4):2657-2672. doi: 10.1039/c9nr07778b. Epub 2020 Jan 15. Nanoscale. 2020. PMID: 31939953 Free PMC article.
-
Unlocking Multimodal Nonlinear Microscopy for Deep-Tissue Imaging under Continuous-Wave Excitation with Tunable Upconverting Nanoparticles.Adv Mater. 2025 May;37(19):e2502739. doi: 10.1002/adma.202502739. Epub 2025 Mar 21. Adv Mater. 2025. PMID: 40116492 Free PMC article.
-
Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, biomolecules, and microspheres.Angew Chem Int Ed Engl. 2013 Mar 25;52(13):3584-600. doi: 10.1002/anie.201208196. Epub 2013 Feb 28. Angew Chem Int Ed Engl. 2013. PMID: 23450698 Review.
-
Compact, Programmable, and Stable Biofunctionalized Upconversion Nanoparticles Prepared through Peptide-Mediated Phase Transfer for High-Sensitive Protease Sensing and in Vivo Apoptosis Imaging.ACS Appl Mater Interfaces. 2015 Jun 10;7(22):11849-56. doi: 10.1021/acsami.5b01446. Epub 2015 Jun 1. ACS Appl Mater Interfaces. 2015. PMID: 25970768
-
Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging.Chem Soc Rev. 2015 Mar 21;44(6):1302-17. doi: 10.1039/c4cs00173g. Chem Soc Rev. 2015. PMID: 25042637 Review.
Cited by
-
Long-wavelength analyte-sensitive luminescent probes and optical (bio)sensors.Methods Appl Fluoresc. 2015 Dec;3(4):042005. doi: 10.1088/2050-6120/3/4/042005. Epub 2015 Oct 22. Methods Appl Fluoresc. 2015. PMID: 27134748 Free PMC article.
-
Near-infrared light activated delivery platform for cancer therapy.Adv Colloid Interface Sci. 2015 Dec;226(Pt B):123-37. doi: 10.1016/j.cis.2015.10.003. Epub 2015 Oct 14. Adv Colloid Interface Sci. 2015. PMID: 26520243 Free PMC article. Review.
-
Photostable and efficient upconverting nanocrystal-based chemical sensors.Opt Mater (Amst). 2018 Oct;84:345-353. doi: 10.1016/j.optmat.2018.07.031. Epub 2018 Jul 17. Opt Mater (Amst). 2018. PMID: 31871387 Free PMC article.
-
Lanthanide nano-drums: a new class of molecular nanoparticles for potential biomedical applications.Faraday Discuss. 2014;175:241-55. doi: 10.1039/c4fd00117f. Faraday Discuss. 2014. PMID: 25284181 Free PMC article.
-
Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis.Regen Eng Transl Med. 2016 Mar;2(1):37-50. doi: 10.1007/s40883-016-0012-9. Epub 2016 Mar 3. Regen Eng Transl Med. 2016. PMID: 27135051 Free PMC article.
References
-
- Chatterjee DK, Rufaihah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008;29(7):937–943. - PubMed
-
- Wang F, Banerjee D, Liu Y, Chen X, Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst (Lond) 2010;135(8):1839–1854. - PubMed
-
- Zhou J, Liu Z, Li F. Upconversion nanophosphors for small-animal imaging. Chem Soc Rev. 2012;41(3):1323–1349. - PubMed
-
- Auzel F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem Rev. 2004;104(1):139–173. - PubMed
-
- Ovsyakin VV, Feofilov PP. Mechanism of summation of electronic excitations in activated crystals. JETP Lett. 1966;3(12):322–327.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

