Neuregulin 1-HER axis as a key mediator of hyperglycemic memory effects in breast cancer
- PMID: 23213231
- PMCID: PMC3529089
- DOI: 10.1073/pnas.1214400109
Neuregulin 1-HER axis as a key mediator of hyperglycemic memory effects in breast cancer
Abstract
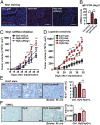
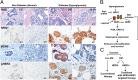
Poor outcomes in diabetic patients are observed across a range of human tumors, suggesting that cancer cells develop unique characteristics under diabetic conditions. Cancer cells exposed to hyperglycemic insults acquire permanent aggressive traits of tumor growth, even after a return to euglycemic conditions. Comparative genome-wide mapping of hyperglycemia-specific open chromatin regions and concomitant mRNA expression profiling revealed that the neuregulin-1 gene, encoding an established endogenous ligand for the HER3 receptor, is activated through a putative distal enhancer. Our findings highlight the targeted inhibition of NRG1-HER3 pathways as a potential target for the treatment breast cancer patients with associated diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. - PubMed
-
- Stattin P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30(3):561–567. - PubMed
-
- Jee SH, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

