G-protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes
- PMID: 23213235
- PMCID: PMC3529095
- DOI: 10.1073/pnas.1205345109
G-protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes
Abstract
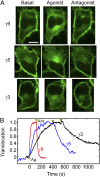
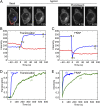
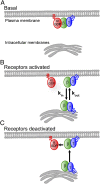
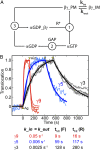
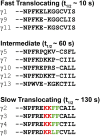
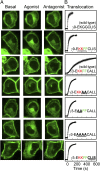
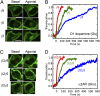
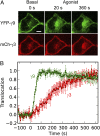
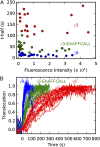
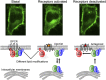
Activation of G-protein heterotrimers by receptors at the plasma membrane stimulates βγ-complex dissociation from the α-subunit and translocation to internal membranes. This intermembrane movement of lipid-modified proteins is a fundamental but poorly understood feature of cell signaling. The differential translocation of G-protein βγ-subunit types provides a valuable experimental model to examine the movement of signaling proteins between membranes in a living cell. We used live cell imaging, mathematical modeling, and in vitro measurements of lipidated fluorescent peptide dissociation from vesicles to determine the mechanistic basis of the intermembrane movement and identify the interactions responsible for differential translocation kinetics in this family of evolutionarily conserved proteins. We found that the reversible translocation is mediated by the limited affinity of the βγ-subunits for membranes. The differential kinetics of the βγ-subunit types are determined by variations among a set of basic and hydrophobic residues in the γ-subunit types. G-protein signaling thus leverages the wide variation in membrane dissociation rates among different γ-subunit types to differentially control βγ-translocation kinetics in response to receptor activation. The conservation of primary structures of γ-subunits across mammalian species suggests that there can be evolutionary selection for primary structures that confer specific membrane-binding affinities and consequent rates of intermembrane movement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

