Essential contribution of IRF3 to intestinal homeostasis and microbiota-mediated Tslp gene induction
- PMID: 23213237
- PMCID: PMC3529020
- DOI: 10.1073/pnas.1219482110
Essential contribution of IRF3 to intestinal homeostasis and microbiota-mediated Tslp gene induction
Abstract
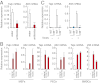
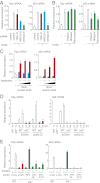
The large intestinal epithelial cells and immune cells are exposed to a variety of molecules derived from commensal microbiota that can activate innate receptors, such as Toll-like receptors (TLRs) and retinoic acid-inducible gene-I-like receptors (RLRs). Although the activation of these receptors is known to be critical for homeostasis of the large intestine, the underlying gene regulatory mechanisms are not well understood. Here, we show that IFN regulatory factor (IRF)3 is critical for the suppression of dextran sulfate sodium-induced colitis. IRF3-deficient mice exhibited lethal defects in the inflammatory and recovery phases of the colitis, accompanied by marked defects in the gene induction for thymic stromal lymphopoietin (TSLP), a cytokine known to be essential for protection of the large intestine. We further provide evidence that DNA and RNA of the large intestinal contents are critical for Tslp gene induction via IRF3 activation by cytosolic nucleic acid receptors. We also demonstrate that IRF3 indeed activates the gene promoter of Tslp via IRF-binding sequences. This newly identified intestinal gene regulatory mechanism, wherein IRF3 activated by microbiota-derived nucleic acids plays a critical role in intestinal homeostasis, may have clinical implication in colonic inflammatory disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

