Complete fiber structures of complex trimeric autotransporter adhesins conserved in enterobacteria
- PMID: 23213248
- PMCID: PMC3529040
- DOI: 10.1073/pnas.1211872110
Complete fiber structures of complex trimeric autotransporter adhesins conserved in enterobacteria
Abstract
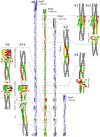
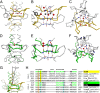
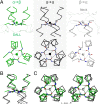
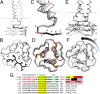
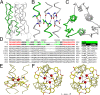
Trimeric autotransporter adhesins (TAAs) are modular, highly repetitive surface proteins that mediate adhesion to host cells in a broad range of Gram-negative pathogens. Although their sizes may differ by more than one order of magnitude, they all follow the same basic head-stalk-anchor architecture, where the head mediates adhesion and autoagglutination, the stalk projects the head from the bacterial surface, and the anchor provides the export function and attaches the adhesin to the bacterial outer membrane after export is complete. In complex adhesins, head and stalk domains may alternate several times before the anchor is reached. Despite extensive sequence divergence, the structures of TAA domains are highly constrained, due to the tight interleaving of their constituent polypeptide chains. We have therefore taken a "domain dictionary" approach to characterize representatives for each domain type by X-ray crystallography and use these structures to reconstruct complete TAA fibers. With SadA from Salmonella enterica, EhaG from enteropathogenic Escherichia coli (EHEC), and UpaG from uropathogenic E. coli (UPEC), we present three representative structures of a complex adhesin that occur in a conserved genomic context in Enterobacteria and is essential in the infection process of uropathogenic E. coli. Our work proves the applicability of the dictionary approach to understanding the structure of a class of proteins that are otherwise poorly tractable by high-resolution methods and provides a basis for the rapid and detailed annotation of newly identified TAAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gerlach RG, Hensel M. Protein secretion systems and adhesins: The molecular armory of Gram-negative pathogens. Int J Med Microbiol. 2007;297(6):401–415. - PubMed
-
- Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA. Trimeric autotransporter adhesins: Variable structure, common function. Trends Microbiol. 2006;14(6):264–270. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

