F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex
- PMID: 23213249
- PMCID: PMC3529094
- DOI: 10.1073/pnas.1214753109
F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex
Abstract
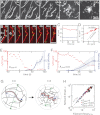
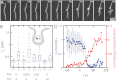
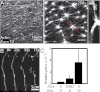
Here we develop a minimal model of the cell actomyosin cortex by forming a quasi-2D cross-linked filamentous actin (F-actin) network adhered to a model cell membrane and contracted by myosin thick filaments. Myosin motors generate both compressive and tensile stresses on F-actin and consequently induce large bending fluctuations, which reduces their effective persistence length to <1 μm. Over a large range of conditions, we show the extent of network contraction corresponds exactly to the extent of individual F-actin shortening via buckling. This demonstrates an essential role of buckling in breaking the symmetry between tensile and compressive stresses to facilitate mesoscale network contraction of up to 80% strain. Portions of buckled F-actin with a radius of curvature ~300 nm are prone to severing and thus compressive stresses mechanically coordinate contractility with F-actin severing, the initial step of F-actin turnover. Finally, the F-actin curvature acquired by myosin-induced stresses can be further constrained by adhesion of the network to a membrane, accelerating filament severing but inhibiting the long-range transmission of the stresses necessary for network contractility. Thus, the extent of membrane adhesion can regulate the coupling between network contraction and F-actin severing. These data demonstrate the essential role of the nonlinear response of F-actin to compressive stresses in potentiating both myosin-mediated contractility and filament severing. This may serve as a general mechanism to mechanically coordinate contractility and cortical dynamics across diverse actomyosin assemblies in smooth muscle and nonmuscle cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bray D, White JG. Cortical flow in animal cells. Science. 1988;239(4842):883–888. - PubMed
-
- Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15(8):732–736. - PubMed
-
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8(3):215–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

