Altered CD38/Cyclic ADP-Ribose Signaling Contributes to the Asthmatic Phenotype
- PMID: 23213344
- PMCID: PMC3508580
- DOI: 10.1155/2012/289468
Altered CD38/Cyclic ADP-Ribose Signaling Contributes to the Asthmatic Phenotype
Abstract
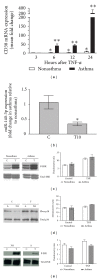
CD38 is a transmembrane glycoprotein expressed in airway smooth muscle cells. The enzymatic activity of CD38 generates cyclic ADP-ribose from β-NAD. Cyclic ADP-ribose mobilizes intracellular calcium during activation of airway smooth muscle cells by G-protein-coupled receptors through activation of ryanodine receptor channels in the sarcoplasmic reticulum. Inflammatory cytokines that are implicated in asthma upregulate CD38 expression and increase the calcium responses to contractile agonists in airway smooth muscle cells. The augmented intracellular calcium responses following cytokine exposure of airway smooth muscle cells are inhibited by an antagonist of cyclic ADP-ribose. Airway smooth muscle cells from CD38 knockout mice exhibit attenuated intracellular calcium responses to agonists, and these mice have reduced airway response to inhaled methacholine. CD38 also contributes to airway hyperresponsiveness as shown in mouse models of allergen or cytokine-induced inflammatory airway disease. In airway smooth muscle cells obtained from asthmatics, the cytokine-induced CD38 expression is significantly enhanced compared to expression in cells from nonasthmatics. This differential induction of CD38 expression in asthmatic airway smooth muscle cells stems from increased activation of MAP kinases and transcription through NF-κB, and altered post-transcriptional regulation through microRNAs. We propose that increased capacity for CD38 signaling in airway smooth muscle in asthma contributes to airway hyperresponsiveness.
Figures
References
-
- Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. American Journal of Physiology. 2005;288(5):L773–L788. - PubMed
-
- Liu X, Farley JM. Depletion and refilling of acetylcholine- and caffeine-sensitive Ca++ stores in tracheal myocytes. Journal of Pharmacology and Experimental Therapeutics. 1996;277(2):789–795. - PubMed
-
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. American Journal of Physiology. 2004;286(5):L909–L917. - PubMed
-
- Sims SM, Jiao Y, Zheng ZG. Intracellular calcium stores in isolated tracheal smooth muscle cells. American Journal of Physiology. 1996;271(2):L300–L309. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

