Transcription factor NF-Y is involved in differentiation of R7 photoreceptor cell in Drosophila
- PMID: 23213364
- PMCID: PMC3507159
- DOI: 10.1242/bio.2011013
Transcription factor NF-Y is involved in differentiation of R7 photoreceptor cell in Drosophila
Abstract
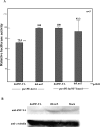
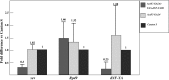
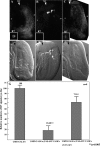
The CCAAT motif-binding factor NF-Y consists of three different subunits, NF-YA, NF-YB and NF-YC. Knockdown of Drosophila NF-YA (dNF-YA) in eye discs with GMR-GAL4 and UAS-dNF-YAIR resulted in a rough eye phenotype and monitoring of differentiation of photoreceptor cells by LacZ expression in seven up-LacZ and deadpan-lacZ enhancer trap lines revealed associated loss of R7 photoreceptor signals. In line with differentiation of R7 being regulated by the sevenless (sev) gene and the MAPK cascade, the rough eye phenotype and loss of R7 signals in dNF-YA-knockdown flies were rescued by expression of the sev gene, or the D-raf gene, a downstream component of the MAPK cascade. The sev gene promoter contains two dNF-Y-binding consensus sequences which play positive roles in promoter activity. In chromatin immunoprecipitation assays with anti-dNF-YA antibody and S2 cells, the sev gene promoter region containing the NF-Y consensus was effectively amplified in immunoprecipitates from transgenic flies by polymerase chain reaction, indicating that dNF-Y is necessary for appropriate sev expression and involved in R7 photoreceptor cell development.
Keywords: MAPK; NF-Y; R7 photoreceptor; chromatin immunoprecipitation; sevenless.
Figures


Similar articles
-
dNF-YB plays dual roles in cell death and cell differentiation during Drosophila eye development.Gene. 2013 May 15;520(2):106-18. doi: 10.1016/j.gene.2013.02.036. Epub 2013 Mar 5. Gene. 2013. PMID: 23470843
-
Transcription factor NF-Y is involved in regulation of the JNK pathway during Drosophila thorax development.Genes Cells. 2008 Feb;13(2):117-30. doi: 10.1111/j.1365-2443.2007.01155.x. Genes Cells. 2008. PMID: 18233955
-
Drosophila transcription factor NF-Y suppresses transcription of the lipase 4 gene, a key gene for lipid storage.Exp Cell Res. 2022 Nov 1;420(1):113307. doi: 10.1016/j.yexcr.2022.113307. Epub 2022 Aug 24. Exp Cell Res. 2022. PMID: 36028059
-
NF-Y in invertebrates.Biochim Biophys Acta Gene Regul Mech. 2017 May;1860(5):630-635. doi: 10.1016/j.bbagrm.2016.10.008. Epub 2016 Oct 26. Biochim Biophys Acta Gene Regul Mech. 2017. PMID: 27793714 Review.
-
Signaling mechanisms in induction of the R7 photoreceptor in the developing Drosophila retina.Bioessays. 1994 Apr;16(4):237-44. doi: 10.1002/bies.950160406. Bioessays. 1994. PMID: 8031300 Review.
Cited by
-
Nuclear transcription factor Y and its roles in cellular processes related to human disease.Am J Cancer Res. 2013 Aug 14;3(4):339-46. eCollection 2013. Am J Cancer Res. 2013. PMID: 23977444 Free PMC article.
-
Digital gene expression approach over multiple RNA-Seq data sets to detect neoblast transcriptional changes in Schmidtea mediterranea.BMC Genomics. 2015 May 8;16(1):361. doi: 10.1186/s12864-015-1533-1. BMC Genomics. 2015. PMID: 25952370 Free PMC article.
-
Cell Cycle Regulation by NF-YC in Drosophila Eye Imaginal Disc: Implications for Synchronization in the Non-Proliferative Region.Int J Mol Sci. 2023 Jul 30;24(15):12203. doi: 10.3390/ijms241512203. Int J Mol Sci. 2023. PMID: 37569581 Free PMC article.
-
The animal nuclear factor Y: an enigmatic and important heterotrimeric transcription factor.Am J Cancer Res. 2018 Jul 1;8(7):1106-1125. eCollection 2018. Am J Cancer Res. 2018. PMID: 30094088 Free PMC article. Review.
-
The NF-Y complex negatively regulates Caenorhabditis elegans tbx-2 expression.Dev Biol. 2013 Oct 1;382(1):38-47. doi: 10.1016/j.ydbio.2013.08.001. Epub 2013 Aug 8. Dev Biol. 2013. PMID: 23933492 Free PMC article.
References
-
- Bhattacharya A., Deng J. M., Zhang Z., Behringer R., Crombrugghe B., Marty S. N. (2003). The B subunit of the CCAAT box binding transcription factor complex (CBF/NF-Y) is essential for early mouse development and cell proliferation. Cancer res. 63, 8167–8172 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

