Spermatogenesis arrest caused by conditional deletion of Hsp90α in adult mice
- PMID: 23213375
- PMCID: PMC3507171
- DOI: 10.1242/bio.2012646
Spermatogenesis arrest caused by conditional deletion of Hsp90α in adult mice
Abstract
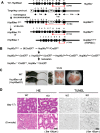
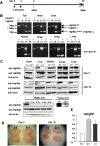
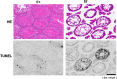
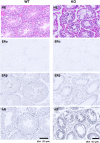
It is controversial whether a functional androgen receptor (AR) on germ cells, including spermatogonia, is essential for their development into sperm and, thus, initiation and maintenance of spermatogenesis. It was recently shown that many spermatocytes underwent apoptosis in the testes of Hsp90α KO mice. We had generated Hsp90α KO mice independently and confirmed this phenotype. However, the important question of whether Hsp90α is required to maintain spermatogenesis in adult mice in which testicular maturation is already completed could not be addressed using these conventional KO mice. To answer this question, we generated a tamoxifen-inducible deletion mutant of Hsp90α and found that conditional deletion of Hsp90α in adult mice caused even more severe apoptosis in germ cells beyond the pachytene stage, leading to complete arrest of spermatogenesis and testicular atrophy. Importantly, immunohistochemical analysis revealed that AR expression in WT testis was more evident in spermatogonia than in spermatocytes, whereas its expression was aberrant and ectopic in Hsp90α KO testis, raising the possibility that an AR abnormality in primordial germ cells is involved in spermatogenesis arrest in the Hsp90α KO mice. Our results suggest that the AR, specifically chaperoned by Hsp90α in spermatogonia, is critical for maintenance of established spermatogenesis and for survival of spermatocytes in adult testis, in addition to setting the first wave of spermatogenesis before puberty.
Keywords: Androgen receptor; Hsp90α; Spermatogenesis; Spermatogonia.
Conflict of interest statement
Figures
Similar articles
-
Dynamics, ultrastructure and gene expression of human in vitro organized testis cells from testicular sperm extraction biopsies.Mol Hum Reprod. 2018 Mar 1;24(3):123-134. doi: 10.1093/molehr/gax070. Mol Hum Reprod. 2018. PMID: 29304256
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
[Expression of androgen receptor and heat shock protein 90alpha in the testicular biopsy specimens of infertile patients with spermatogenic arrest].Zhonghua Nan Ke Xue. 2004 Sep;10(9):662-6. Zhonghua Nan Ke Xue. 2004. PMID: 15497705 Chinese.
-
Loss of connexin 43 in Sertoli cells provokes postnatal spermatogonial arrest, reduced germ cell numbers and impaired spermatogenesis.Reprod Biol. 2018 Dec;18(4):456-466. doi: 10.1016/j.repbio.2018.08.001. Epub 2018 Sep 19. Reprod Biol. 2018. PMID: 30243528
-
Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes.Microsc Res Tech. 2010 Apr;73(4):241-78. doi: 10.1002/jemt.20783. Microsc Res Tech. 2010. PMID: 19941293 Review.
Cited by
-
Hsp90α and Hsp90β together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing.J Cell Sci. 2015 Apr 15;128(8):1475-80. doi: 10.1242/jcs.166363. Epub 2015 Mar 3. J Cell Sci. 2015. PMID: 25736295 Free PMC article.
-
Impact of Heat Stress on Bovine Sperm Quality and Competence.Animals (Basel). 2022 Apr 9;12(8):975. doi: 10.3390/ani12080975. Animals (Basel). 2022. PMID: 35454222 Free PMC article. Review.
-
Polyubiquitin gene Ubb is required for upregulation of Piwi protein level during mouse testis development.Cell Death Discov. 2021 Jul 26;7(1):194. doi: 10.1038/s41420-021-00581-2. Cell Death Discov. 2021. PMID: 34312369 Free PMC article.
-
Gene expression and functional analysis of Aha1a and Aha1b in stress response in zebrafish.Comp Biochem Physiol B Biochem Mol Biol. 2022 Oct-Dec;262:110777. doi: 10.1016/j.cbpb.2022.110777. Epub 2022 Jul 10. Comp Biochem Physiol B Biochem Mol Biol. 2022. PMID: 35830921 Free PMC article.
-
Regulation and function of the human HSP90AA1 gene.Gene. 2015 Oct 1;570(1):8-16. doi: 10.1016/j.gene.2015.06.018. Epub 2015 Jun 10. Gene. 2015. PMID: 26071189 Free PMC article. Review.
References
-
- Chang C., Chen Y. T., Yeh S. D., Xu Q., Wang R. S., Guillou F., Lardy H., Yeh S. (2004). Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 101, 6876–6881 10.1073/pnas.0307306101 - DOI - PMC - PubMed
-
- De Gendt K., Swinnen J. V., Saunders P. T., Schoonjans L., Dewerchin M., Devos A., Tan K., Atanassova N., Claessens F., Lécureuil C.et al. (2004). A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 101, 1327–1332 10.1073/pnas.0308114100 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

