Drosophila sosie functions with β(H)-Spectrin and actin organizers in cell migration, epithelial morphogenesis and cortical stability
- PMID: 23213377
- PMCID: PMC3507177
- DOI: 10.1242/bio.20122154
Drosophila sosie functions with β(H)-Spectrin and actin organizers in cell migration, epithelial morphogenesis and cortical stability
Abstract
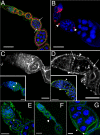
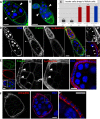
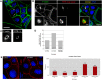
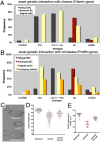
Morphogenesis in multicellular organisms requires the careful coordination of cytoskeletal elements, dynamic regulation of cell adhesion and extensive cell migration. sosie (sie) is a novel gene required in various morphogenesis processes in Drosophila oogenesis. Lack of sie interferes with normal egg chamber packaging, maintenance of epithelial integrity and control of follicle cell migration, indicating that sie is involved in controlling epithelial integrity and cell migration. For these functions sie is required both in the germ line and in the soma. Consistent with this, Sosie localizes to plasma membranes in the germ line and in the somatic follicle cells and is predicted to present an EGF-like domain on the extracellular side. Two positively charged residues, C-terminal to the predicted transmembrane domain (on the cytoplasmic side), are required for normal plasma membrane localization of Sosie. Because sie also contributes to normal cortical localization of β(H)-Spectrin, it appears that cortical β(H)-Spectrin mediates some of the functions of sosie. sie also interacts with the genes coding for the actin organizers Filamin and Profilin and, in the absence of sie function, F-actin is less well organized and nurse cells frequently fuse.
Keywords: Cell migration; Cortical organization and stability; Drosophila oogenesis; Epithelia; Morphogenesis; Spectrin.
Conflict of interest statement
Figures



References
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

