Lhx1 in the proximal region of the optic vesicle permits neural retina development in the chicken
- PMID: 23213388
- PMCID: PMC3507191
- DOI: 10.1242/bio.20121396
Lhx1 in the proximal region of the optic vesicle permits neural retina development in the chicken
Abstract
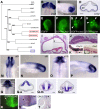
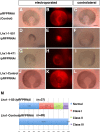
How the eye forms has been one of the fundamental issues in developmental biology. The retinal anlage first appears as the optic vesicle (OV) evaginating from the forebrain. Subsequently, its distal portion invaginates to form the two-walled optic cup, which develops into the outer pigmented and inner neurosensory layers of the retina. Recent work has shown that this optic-cup morphogenesis proceeds as a self-organizing activity without any extrinsic molecules. However, intrinsic factors that regulate this process have not been elucidated. Here we show that a LIM-homeobox gene, Lhx1, normally expressed in the proximal region of the nascent OV, induces a second neurosensory retina formation from the outer pigmented retina when overexpressed in the chicken OV. Lhx2, another LIM-homeobox gene supposed to be involved in early OV formation, could not substitute this function of Lhx1, while Lhx5, closely related to Lhx1, could replace it. Conversely, knockdown of Lhx1 expression by RNA interference resulted in the formation of a small or pigmented vesicle. These results suggest that the proximal region demarcated by Lhx1 expression permits OV development, eventually dividing the two retinal domains.
Keywords: Chicken; Eye development; LIM-homeobox; Lhx1; Lhx5; Neural retina; Optic vesicle.
Conflict of interest statement
Figures






References
-
- Das R. M., Van Hateren N. J., Howell G. R., Farrell E. R., Bangs F. K., Porteous V. C., Manning E. M., McGrew M. J., Ohyama K., Sacco M. A.et al. (2006). A robust system for RNA interference in the chicken using a modified microRNA operon. Dev. Biol. 294, 554–563 10.1016/j.ydbio.2006.02.020 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

