A role for Rap2 in recycling the extended conformation of LFA-1 during T cell migration
- PMID: 23213397
- PMCID: PMC3507183
- DOI: 10.1242/bio.20122824
A role for Rap2 in recycling the extended conformation of LFA-1 during T cell migration
Abstract
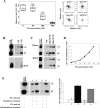
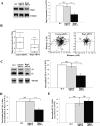
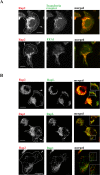
T lymphocytes make use of their major integrin LFA-1 to migrate on surfaces that express ICAM-1 such as blood vessels and inflamed tissue sites. How the adhesions are turned over in order to supply traction for this migration has not been extensively investigated. By following the fate of biotinylated membrane LFA-1 on T lymphocytes, we show in this study that LFA-1 internalization and re-exposure on the plasma membrane are linked to migration. Previously we demonstrated the GTPase Rap2 to be a regulator of LFA-1-mediated migration. SiRNA knockdown of this GTPase inhibits both LFA-1 internalization and also its ability to be re-exposed, indicating that Rap2 participates in recycling of LFA-1 and influences its complete endocytosis-exocytosis cycle. Confocal microscopy images reveal that the intracellular distribution of Rap2 overlaps with endosomal recycling vesicles. Although the homologous GTPase Rap1 is also found on intracellular vesicles and associated with LFA-1 activation, these two homologous GTPases do not co-localize. Little is known about the conformation of the LFA-1 that is recycled. We show that the extended form of LFA-1 is internalized and in Rap2 siRNA-treated T lymphocytes the trafficking of this LFA-1 conformation is disrupted resulting in its intracellular accumulation. Thus LFA-1-mediated migration of T lymphocytes requires Rap2-expressing vesicles to recycle the extended form of LFA-1 that we have previously found to control migration at the leading edge.
Keywords: Integrin; LFA-1; Migration; Rap2; Recycling.
Conflict of interest statement
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

