Wnt signalling antagonizes stress granule assembly through a Dishevelled-dependent mechanism
- PMID: 23213403
- PMCID: PMC3507204
- DOI: 10.1242/bio.2011023
Wnt signalling antagonizes stress granule assembly through a Dishevelled-dependent mechanism
Abstract
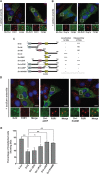
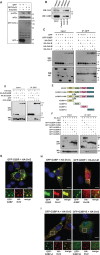
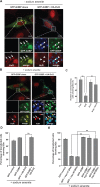
Cells often respond to diverse environmental stresses by inducing stress granules (SGs) as an adaptive mechanism. SGs are generally assembled as a result of aggregation of mRNAs stalled in a translational pre-initiation complex, mediated by a set of RNA-binding proteins such as G3BP and TIA-1. SGs may serve as triage centres for storage, translation re-initiation or degradation of specific mRNAs. However, the mechanism involved in the modulation of their assembly/disassembly is unclear. Here we report that Wnt signalling negatively regulates SG assembly through Dishevelled (Dvl), a cytoplasmic Wnt effector. Overexpression of Dvl2, an isoform of Dvl, leads to impairment of SG assembly through a DEP domain dependent mechanism. Intriguingly, the Dvl2 mutant K446M, which corresponds to an analogous mutation in Drosophila Dishevelled DEP domain (dsh(1)) that results in defective PCP pathway, fails to antagonize SG assembly. Furthermore, we show that Dvl2 exerts the antagonistic effect on SG assembly through a mechanism involving Rac1-mediated inhibition of RhoA. Dvl2 interacts with G3BP, a downstream component of Ras signalling involved in SG assembly, and functional analysis suggests a model wherein the Dvl-Rac1-RhoA axis regulates G3BP's SG-nucleating activity. Collectively, these results define an antagonistic effect of Wnt signalling on SG assembly, and reveal a novel role for Wnt/Dvl pathway in the modulation of mRNA functions.
Keywords: Dvl; G3BP; RNA; Stress granule; Wnt.
Figures


Similar articles
-
The RasGAP-associated endoribonuclease G3BP mediates stress granule assembly.J Cell Biol. 2023 Nov 6;222(11):e200212128072023new. doi: 10.1083/jcb.200212128072023new. Epub 2023 Sep 6. J Cell Biol. 2023. PMID: 37672657 Free PMC article.
-
The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein.Mol Cell Biol. 2012 Oct;32(19):3903-12. doi: 10.1128/MCB.00251-12. Epub 2012 Jul 23. Mol Cell Biol. 2012. PMID: 22826439 Free PMC article.
-
Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP.Mol Neurodegener. 2012 Oct 24;7:54. doi: 10.1186/1750-1326-7-54. Mol Neurodegener. 2012. PMID: 23092511 Free PMC article.
-
Role of the Ubiquitin System in Stress Granule Metabolism.Int J Mol Sci. 2022 Mar 26;23(7):3624. doi: 10.3390/ijms23073624. Int J Mol Sci. 2022. PMID: 35408984 Free PMC article. Review.
-
Dishevelled: The hub of Wnt signaling.Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review.
Cited by
-
NSCLC cells sustain phase separation of cytoplasmic membrane-less organelles to protect themselves against cisplatin treatment.Acta Pharmacol Sin. 2025 Sep;46(9):2522-2533. doi: 10.1038/s41401-025-01551-5. Epub 2025 Apr 17. Acta Pharmacol Sin. 2025. PMID: 40247038
-
A GTPase-activating protein-binding protein (G3BP1)/antiviral protein relay conveys arteriosclerotic Wnt signals in aortic smooth muscle cells.J Biol Chem. 2018 May 25;293(21):7942-7968. doi: 10.1074/jbc.RA118.002046. Epub 2018 Apr 6. J Biol Chem. 2018. PMID: 29626090 Free PMC article.
-
The link between Wnt-related, stress-related, and circadian genes in the dermal fibroblasts of individuals with attention-deficit hyperactivity disorder.J Neural Transm (Vienna). 2025 Jul 21. doi: 10.1007/s00702-025-02986-0. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40690007
-
Zebrafish P54 RNA helicases are cytoplasmic granule residents that are required for development and stress resilience.Biol Open. 2016 Oct 15;5(10):1473-1484. doi: 10.1242/bio.015826. Biol Open. 2016. PMID: 27489304 Free PMC article.
-
Nup358 interacts with Dishevelled and aPKC to regulate neuronal polarity.Biol Open. 2013 Oct 16;2(11):1270-8. doi: 10.1242/bio.20135363. eCollection 2013. Biol Open. 2013. PMID: 24244865 Free PMC article.
References
-
- Almeida M., Han L., Martin-Millan M., O'Brien C. A., Manolagas S. C. (2007). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 282, 27, 298–27, 305 10.1074/jbc.M70281120 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

