Sumoylation is tumor-suppressive and confers proliferative quiescence to hematopoietic progenitors in Drosophila melanogaster larvae
- PMID: 23213407
- PMCID: PMC3507282
- DOI: 10.1242/bio.2012043
Sumoylation is tumor-suppressive and confers proliferative quiescence to hematopoietic progenitors in Drosophila melanogaster larvae
Abstract
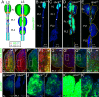
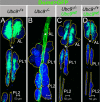
How cell-intrinsic regulation of the cell cycle and the extrinsic influence of the niche converge to provide proliferative quiescence, safeguard tissue integrity, and provide avenues to stop stem cells from giving rise to tumors is a major challenge in gene therapy and tissue engineering. We explore this question in sumoylation-deficient mutants of Drosophila. In wild type third instar larval lymph glands, a group of hematopoietic stem/progenitor cells acquires quiescence; a multicellular niche supports their undifferentiated state. However, how proliferative quiescence is instilled in this population is not understood. We show that Ubc9 protein is nuclear in this population. Loss of the SUMO-activating E1 enzyme, Aos1/Uba2, the conjugating E2 enzyme, Ubc9, or the E3 SUMO ligase, PIAS, results in a failure of progenitors to quiesce; progenitors become hyperplastic, misdifferentiate, and develop into microtumors that eventually detach from the dorsal vessel. Significantly, dysplasia and lethality of Ubc9 mutants are rescued when Ubc9(wt) is provided specifically in the progenitor populations, but not when it is provided in the niche or in the differentiated cortex. While normal progenitors express high levels of the Drosophila cyclin-dependent kinase inhibitor p21 homolog, Dacapo, the corresponding overgrown mutant population exhibits a marked reduction in Dacapo. Forced expression of either Dacapo or human p21 in progenitors shrinks this population. The selective expression of either protein in mutant progenitor cells, but not in other hematopoietic populations, limits overgrowth, blocks tumorogenesis, and restores organ integrity. We discuss an essential and complex role for sumoylation in preserving the hematopoietic progenitor states for stress response and in the context of normal development of the fly.
Keywords: Dacapo; Ubc9; dysplasia; hematopoiesis; microtumor; niche; organ integrity; p21; quiescence; stem cell; sumoylation; tumor suppressor.
Figures





References
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

