Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors
- PMID: 23213423
- PMCID: PMC3509452
- DOI: 10.1242/bio.2012364
Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors
Abstract
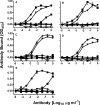
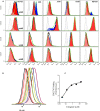
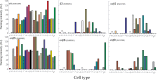
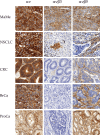
The relationship between integrin expression and function in pathologies is often contentious as comparisons between human pathological expression and expression in cell lines is difficult. In addition, the expression of even integrins αvβ6 and αvβ8 in tumor cell lines is not comprehensively documented. Here, we describe rabbit monoclonal antibodies (RabMabs) against the extracellular domains of αv integrins that react with both native integrins and formalin fixed, paraffin embedded (FFPE) human tissues. These RabMabs, against αvβ3 (EM22703), αvβ5 (EM09902), αvβ6 (EM05201), αvβ8 (EM13309), and pan-αv (EM01309), recognize individual integrin chains in Western blots and in flow cytometry. EM22703 detected a ligand-induced binding site (LIBS), reporting an epitope enhanced by the binding of an RGD-peptide to αvβ3. αvβ8 was rarely expressed in human tumor specimens, and weakly expressed in non-small-cell lung carcinoma (NSCLC). However, ovarian carcinoma cell lines expressed αvβ8, as did some melanoma cells, whereas U87MG glioma lacked αvβ8 expression. We observed an unexpected strong expression of αvβ6 in tumor samples of invasive ductal breast adenoma, colorectal carcinoma (CRC), and NSCLC. αvβ3 was strongly expressed in some invasive NSCLC cohorts. Interestingly, PC3 prostate cell and human prostate tumors did not express αvβ3. The RabMabs stained plasma membranes in FFPE-immunohistochemistry (IHC) samples of tumor cell lines from lung, ovary, colon, prostate, squamous cell carcinoma of head and neck (SCCHN), breast, and pancreas carcinomas. The RabMabs are unique tools for probing αv integrin biology, and suggest that especially αvβ6 and αvβ8 biologies still have much to reveal.
Keywords: Alphav; Immunohistology; Integrin; Paraffin embedded; Rabbit-monoclonal.
Conflict of interest statement
Figures




Similar articles
-
Validation and comparison of anti-αvβ3 and anti-αvβ5 rabbit monoclonal versus murine monoclonal antibodies in four different tumor entities.Appl Immunohistochem Mol Morphol. 2013 Dec;21(6):553-60. doi: 10.1097/PAI.0b013e318284a03a. Appl Immunohistochem Mol Morphol. 2013. PMID: 23455183
-
Comparing the expression of integrins αvβ3, αvβ5, αvβ6, αvβ8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors.Int J Clin Exp Pathol. 2013 Nov 15;6(12):2719-32. eCollection 2013. Int J Clin Exp Pathol. 2013. PMID: 24294359 Free PMC article.
-
Longitudinal expression analysis of αv integrins in human gliomas reveals upregulation of integrin αvβ3 as a negative prognostic factor.J Neuropathol Exp Neurol. 2013 Mar;72(3):194-210. doi: 10.1097/NEN.0b013e3182851019. J Neuropathol Exp Neurol. 2013. PMID: 23399898
-
RGD-Binding Integrins in Head and Neck Cancers.Cancers (Basel). 2017 May 26;9(6):56. doi: 10.3390/cancers9060056. Cancers (Basel). 2017. PMID: 28587135 Free PMC article. Review.
-
There is a world beyond αvβ3-integrin: Multimeric ligands for imaging of the integrin subtypes αvβ6, αvβ8, αvβ3, and α5β1 by positron emission tomography.EJNMMI Res. 2021 Oct 12;11(1):106. doi: 10.1186/s13550-021-00842-2. EJNMMI Res. 2021. PMID: 34636990 Free PMC article. Review.
Cited by
-
Analysis of differentially expressed genes, clinical value and biological pathways in prostate cancer.Am J Transl Res. 2018 May 15;10(5):1444-1456. eCollection 2018. Am J Transl Res. 2018. PMID: 29887958 Free PMC article.
-
K562 Chronic Myeloid Leukemia Cells as a Dual β3-Expressing Functional Cell Line Model to Investigate the Effects of Combined αIIbβ3 and αvβ3 Antagonism.Methods Protoc. 2025 Jul 5;8(4):73. doi: 10.3390/mps8040073. Methods Protoc. 2025. PMID: 40700311 Free PMC article.
-
The functional role of integrins during intra- and extravasation within the metastatic cascade.Mol Cancer. 2019 Jan 18;18(1):12. doi: 10.1186/s12943-018-0937-3. Mol Cancer. 2019. PMID: 30657059 Free PMC article. Review.
-
Expression Analysis of α5 Integrin Subunit Reveals Its Upregulation as a Negative Prognostic Biomarker for Glioblastoma.Pharmaceuticals (Basel). 2021 Aug 30;14(9):882. doi: 10.3390/ph14090882. Pharmaceuticals (Basel). 2021. PMID: 34577582 Free PMC article.
-
Cytoskeletal Proteins in Cancer and Intracellular Stress: A Therapeutic Perspective.Cancers (Basel). 2020 Jan 18;12(1):238. doi: 10.3390/cancers12010238. Cancers (Basel). 2020. PMID: 31963677 Free PMC article. Review.
References
-
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. (1990). Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 50, 6757–6764. - PubMed
-
- Albert J. M., Cao C., Geng L., Leavitt L., Hallahan D. E., Lu B. (2006). Integrin alpha v beta 3 antagonist Cilengitide enhances efficacy of radiotherapy in endothelial cell and non-small-cell lung cancer models. Int. J. Radiat. Oncol. Biol. Phys. 65, 1536–1543 10.1016/j.ijrobp.2006.04.036 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

