Connexin26-mediated transfer of laterality cues in Xenopus
- PMID: 23213439
- PMCID: PMC3507211
- DOI: 10.1242/bio.2012760
Connexin26-mediated transfer of laterality cues in Xenopus
Abstract
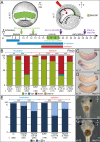
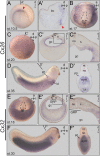
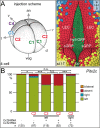
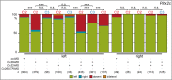
A cilia-driven leftward flow of extracellular fluid breaks bilateral symmetry in the dorsal midline of the neurula stage vertebrate embryo. The left-specific Nodal signaling cascade in the lateral plate mesoderm (LPM) is key to asymmetric morphogenesis and placement of organs during subsequent development. The nature of the initial asymmetric cue(s) as well as the transfer of information from the midline to the left side has remained elusive. Gap junctional communication has been previously involved in Xenopus left-right (LR) development, however a function at cleavage stages was inferred from inhibitor experiments. Here we show by heptanol-mediated block of connexin function that flow stages during neurulation represent the critical time window. Flow in Xenopus occurs at the gastrocoel roof plate (GRP), a ciliated sheath of cells of mesodermal fate transiently positioned within the dorsal epithelial lining of the forming archenteron. We reasoned that endodermal cells immediately adjacent to the GRP are important for transfer of asymmetry. A systematic screen identified two connexin genes, Cx26 and Cx32, which were co-expressed in these lateral endodermal cells. Gain- and loss-of-function experiments pinpointed Cx26 as the critical connexin for LR development, while Cx32 had no effect on laterality. Importantly, GRP morphology, ciliation and flow were not affected in Cx26 morphants. Our results demonstrate a decisive role of Cx26 in the transfer of laterality cues from the GRP to the left LPM, providing a novel access to the identification of the initial asymmetric signal generated by flow.
Keywords: Connexin; Cx26; Cx32; Gap junctional communication; Gap junctions; Left-right asymmetry; Xenopus laevis.
Conflict of interest statement
Figures


Similar articles
-
The nodal inhibitor Coco is a critical target of leftward flow in Xenopus.Curr Biol. 2010 Apr 27;20(8):738-43. doi: 10.1016/j.cub.2010.02.061. Epub 2010 Apr 8. Curr Biol. 2010. PMID: 20381352
-
Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis.Dev Biol. 2009 Jul 15;331(2):281-91. doi: 10.1016/j.ydbio.2009.05.547. Epub 2009 May 18. Dev Biol. 2009. PMID: 19450574
-
Cilia-driven leftward flow determines laterality in Xenopus.Curr Biol. 2007 Jan 9;17(1):60-6. doi: 10.1016/j.cub.2006.10.067. Curr Biol. 2007. PMID: 17208188
-
Xenopus, an ideal model system to study vertebrate left-right asymmetry.Dev Dyn. 2009 Jun;238(6):1215-25. doi: 10.1002/dvdy.21855. Dev Dyn. 2009. PMID: 19208433 Review.
-
Linking early determinants and cilia-driven leftward flow in left-right axis specification of Xenopus laevis: a theoretical approach.Differentiation. 2012 Feb;83(2):S67-77. doi: 10.1016/j.diff.2011.11.005. Epub 2011 Dec 1. Differentiation. 2012. PMID: 22136958 Review.
Cited by
-
GJA1 depletion causes ciliary defects by affecting Rab11 trafficking to the ciliary base.Elife. 2022 Aug 25;11:e81016. doi: 10.7554/eLife.81016. Elife. 2022. PMID: 36004726 Free PMC article.
-
Making and breaking symmetry in development, growth and disease.Development. 2019 Aug 15;146(16):dev170985. doi: 10.1242/dev.170985. Development. 2019. PMID: 31416929 Free PMC article. Review.
-
Vertebrate Left-Right Asymmetry: What Can Nodal Cascade Gene Expression Patterns Tell Us?J Cardiovasc Dev Dis. 2017 Dec 29;5(1):1. doi: 10.3390/jcdd5010001. J Cardiovasc Dev Dis. 2017. PMID: 29367579 Free PMC article. Review.
-
A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality.Dev Biol. 2013 Jul 1;379(1):1-15. doi: 10.1016/j.ydbio.2013.03.021. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583583 Free PMC article. Review.
-
Symmetry breakage in the vertebrate embryo: when does it happen and how does it work?Dev Biol. 2014 Sep 1;393(1):109-23. doi: 10.1016/j.ydbio.2014.06.014. Epub 2014 Jun 24. Dev Biol. 2014. PMID: 24972089 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

