Naïve adult stem cells from patients with Hutchinson-Gilford progeria syndrome express low levels of progerin in vivo
- PMID: 23213444
- PMCID: PMC3509444
- DOI: 10.1242/bio.20121149
Naïve adult stem cells from patients with Hutchinson-Gilford progeria syndrome express low levels of progerin in vivo
Abstract
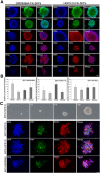
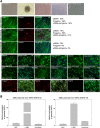
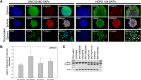
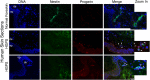
Hutchinson-Gilford progeria syndrome (HGPS, OMIM 176670) is a rare disorder characterized by segmental accelerated aging and early death from coronary artery disease or stroke. Nearly 90% of HGPS sufferers carry a G608G mutation within exon 11 of LMNA, producing a truncated form of prelamin A, referred to as "progerin". Here, we report the isolation of naïve multipotent skin-derived precursor (SKP) cells from dermal fibroblast cultures from HGPS donors. These cells form spheres and express the neural crest marker, nestin, in addition to the multipotent markers, OCT4, Sox2, Nanog and TG30; these cells can self-renew and differentiate into smooth muscle cells (SMCs) and fibroblasts. The SMCs derived from the HGPS-SKPs accumulate nuclear progerin with increasing passages. A subset of the HGPS-naïve SKPs express progerin in vitro and in situ in HGPS skin sections. This is the first in vivo evidence that progerin is produced in adult stem cells, and implies that this protein could induce stem cells exhaustion as a mechanism contributing to aging. Our study provides a basis on which to explore therapeutic applications for HGPS stem cells and opens avenues for investigating the pathogenesis of other genetic diseases.
Keywords: Adult stem cells; HGPS; Lamin A; Progeria; Progerin.
Conflict of interest statement
Figures


Similar articles
-
Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture.J Proteomics. 2013 Oct 8;91:466-77. doi: 10.1016/j.jprot.2013.08.008. Epub 2013 Aug 20. J Proteomics. 2013. PMID: 23969228
-
Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts.Aging Cell. 2015 Feb;14(1):78-91. doi: 10.1111/acel.12300. Epub 2014 Dec 16. Aging Cell. 2015. PMID: 25510262 Free PMC article.
-
Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson-Gilford progeria syndrome.Med Hypotheses. 2018 Sep;118:151-162. doi: 10.1016/j.mehy.2018.06.029. Epub 2018 Jun 28. Med Hypotheses. 2018. PMID: 30037605
-
Hutchinson-Gilford Progeria Syndrome (Hgps) and Application of Gene Therapy Based Crispr/Cas Technology as A Promising Innovative Treatment Approach.Recent Pat Biotechnol. 2021;15(4):266-285. doi: 10.2174/1872208315666210928114720. Recent Pat Biotechnol. 2021. PMID: 34602042 Review.
-
Are There Common Mechanisms Between the Hutchinson-Gilford Progeria Syndrome and Natural Aging?Front Genet. 2019 May 15;10:455. doi: 10.3389/fgene.2019.00455. eCollection 2019. Front Genet. 2019. PMID: 31156709 Free PMC article. Review.
Cited by
-
Giant Panda (Ailuropoda melanoleuca) Buccal Mucosa Tissue as a Source of Multipotent Progenitor Cells.PLoS One. 2015 Sep 23;10(9):e0138840. doi: 10.1371/journal.pone.0138840. eCollection 2015. PLoS One. 2015. PMID: 26398672 Free PMC article.
-
Alterations to Genome Organisation in Stem Cells, Their Differentiation and Associated Diseases.Results Probl Cell Differ. 2022;70:71-102. doi: 10.1007/978-3-031-06573-6_3. Results Probl Cell Differ. 2022. PMID: 36348105
-
Adult Stem Cells and Diseases of Aging.J Clin Med. 2014 Jan 21;3(1):88-134. doi: 10.3390/jcm3010088. J Clin Med. 2014. PMID: 24757526 Free PMC article.
-
Molecular insights into the premature aging disease progeria.Histochem Cell Biol. 2016 Apr;145(4):401-17. doi: 10.1007/s00418-016-1411-1. Epub 2016 Feb 4. Histochem Cell Biol. 2016. PMID: 26847180 Free PMC article. Review.
-
Cells, growth factors and biomaterials used in tissue engineering for hair follicles regeneration.Regen Ther. 2022 Nov 25;21:596-610. doi: 10.1016/j.reth.2022.11.003. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36475027 Free PMC article. Review.
References
-
- Agrelo R., Setien F., Espada J., Artiga M. J., Rodriguez M., Pérez-Rosado A., Sanchez-Aguilera A., Fraga M. F., Piris M. A., Esteller M. (2005). Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J. Clin. Oncol. 23, 3940–3947 10.1200/JCO.2005.11.650 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

