CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte
- PMID: 23213457
- PMCID: PMC3507294
- DOI: 10.1242/bio.20121420
CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte
Abstract
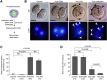
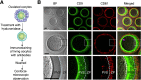
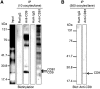
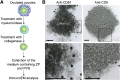
When a sperm and oocyte unite into one cell upon fertilization, membranous fusion between the sperm and oocyte occurs. In mice, Izumo1 and a tetraspanin molecule CD9 are required for sperm-oocyte fusion as one of the oocyte factors, and another tetraspanin molecule CD81 is also thought to involve in this process. Since these two tetraspanins often form a complex upon cell-cell interaction, it is probable that such a complex is also formed in sperm-oocyte interaction; however, this possibility is still under debate among researchers. Here we assessed this problem using mouse oocytes. Immunocytochemical analysis demonstrated that both CD9 and CD81 were widely distributed outside the oocyte cell membrane, but these molecules were separate, forming bilayers, confirmed by immunobiochemical analysis. Electron-microscopic analysis revealed the presence of CD9- or CD81-incorporated extracellular structures in those bilayers. Finally, microinjection of in vitro-synthesized RNA showed that CD9 reversed a fusion defect in CD81-deficient oocytes in addition to CD9-deficient oocytes, but CD81 failed in both oocytes. These results suggest that both CD9 and CD81 independently work upon sperm-oocyte fusion as extracellular components.
Keywords: CD81; CD9; Exosome; Membrane fusion.
Conflict of interest statement
Figures


Similar articles
-
Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion.Dev Biol. 2002 Jul 15;247(2):327-34. doi: 10.1006/dbio.2002.0694. Dev Biol. 2002. PMID: 12086470
-
Reduced fertility of female mice lacking CD81.Dev Biol. 2006 Feb 15;290(2):351-8. doi: 10.1016/j.ydbio.2005.11.031. Epub 2005 Dec 27. Dev Biol. 2006. PMID: 16380109
-
CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization.Int J Mol Sci. 2018 Apr 19;19(4):1236. doi: 10.3390/ijms19041236. Int J Mol Sci. 2018. PMID: 29671763 Free PMC article.
-
Role of tetraspanin CD9 molecule in fertilization of mammals.Physiol Res. 2015;64(3):279-93. doi: 10.33549/physiolres.932876. Epub 2014 Dec 22. Physiol Res. 2015. PMID: 25536312 Review.
-
Tetraspanins in mammalian reproduction: spermatozoa, oocytes and embryos.Med Microbiol Immunol. 2020 Aug;209(4):407-425. doi: 10.1007/s00430-020-00676-0. Epub 2020 May 18. Med Microbiol Immunol. 2020. PMID: 32424440 Review.
Cited by
-
Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction.Int J Mol Sci. 2020 Oct 14;21(20):7568. doi: 10.3390/ijms21207568. Int J Mol Sci. 2020. PMID: 33066349 Free PMC article. Review.
-
Stem cell treatments for female reproductive disorders: a comprehensive review.J Ovarian Res. 2025 Jul 24;18(1):161. doi: 10.1186/s13048-025-01750-y. J Ovarian Res. 2025. PMID: 40708035 Free PMC article. Review.
-
Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption.Mol Reprod Dev. 2013 Apr;80(4):260-72. doi: 10.1002/mrd.22160. Epub 2013 Mar 13. Mol Reprod Dev. 2013. PMID: 23401167 Free PMC article.
-
Proteomic and Bioinformatic Analysis of Decellularized Pancreatic Extracellular Matrices.Molecules. 2021 Nov 8;26(21):6740. doi: 10.3390/molecules26216740. Molecules. 2021. PMID: 34771149 Free PMC article.
-
Sperm-egg fusion: a molecular enigma of mammalian reproduction.Int J Mol Sci. 2014 Jun 13;15(6):10652-68. doi: 10.3390/ijms150610652. Int J Mol Sci. 2014. PMID: 24933635 Free PMC article. Review.
References
-
- Chen M. S., Tung K. S., Coonrod S. A., Takahashi Y., Bigler D., Chang A., Yamashita Y., Kincade P. W., Herr J. C., White J. M. (1999). Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc. Natl. Acad. Sci. USA 96, 11830–11835 10.1073/pnas.96.21.11830 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

