The loss of Hoxa5 function promotes Notch-dependent goblet cell metaplasia in lung airways
- PMID: 23213461
- PMCID: PMC3507293
- DOI: 10.1242/bio.20121701
The loss of Hoxa5 function promotes Notch-dependent goblet cell metaplasia in lung airways
Abstract
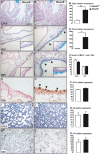
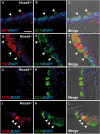
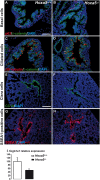
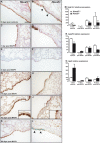
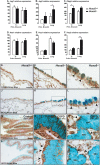
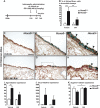
Hox genes encode transcription factors controlling complex developmental processes in various organs. Little is known, however, about how HOX proteins control cell fate. Herein, we demonstrate that the goblet cell metaplasia observed in lung airways from Hoxa5(-/-) mice originates from the transdifferentiation of Clara cells. Reduced CC10 expression in Hoxa5(-/-) embryos indicates that altered cell specification occurs prior to birth. The loss of Hoxa5 function does not preclude airway repair after naphthalene exposure, but the regenerated epithelium presents goblet cell metaplasia and less CC10-positive cells, demonstrating the essential role of Hoxa5 for correct differentiation. Goblet cell metaplasia in Hoxa5(-/-) mice is a FOXA2-independent process. However, it is associated with increased Notch signaling activity. Consistent with these findings, expression levels of activated NOTCH1 and the effector gene HEY2 are enhanced in patients with chronic obstructive pulmonary disease. In vivo administration of a γ-secretase inhibitor attenuates goblet cell metaplasia in Hoxa5(-/-) mice, highlighting the contribution of Notch signaling to the phenotype and suggesting a potential therapeutic strategy to inhibit goblet cell differentiation and mucus overproduction in airway diseases. In summary, the loss of Hoxa5 function in lung mesenchyme impacts on epithelial cell fate by modulating Notch signaling.
Keywords: Goblet cells; Hox genes; Notch pathway.
Conflict of interest statement
Figures



Similar articles
-
Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development.Development. 2011 Aug;138(16):3533-43. doi: 10.1242/dev.063727. Development. 2011. PMID: 21791528 Free PMC article.
-
Partial functional redundancy between Hoxa5 and Hoxb5 paralog genes during lung morphogenesis.Am J Physiol Lung Cell Mol Physiol. 2013 Jun 15;304(12):L817-30. doi: 10.1152/ajplung.00006.2013. Epub 2013 Apr 12. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23585229 Free PMC article.
-
Antisense Oligonucleotides Targeting Jagged 1 Reduce House Dust Mite-induced Goblet Cell Metaplasia in the Adult Murine Lung.Am J Respir Cell Mol Biol. 2020 Jul;63(1):46-56. doi: 10.1165/rcmb.2019-0257OC. Am J Respir Cell Mol Biol. 2020. PMID: 32176858
-
[Hoxa5: a master gene with multifaceted roles].Med Sci (Paris). 2009 Jan;25(1):77-82. doi: 10.1051/medsci/200925177. Med Sci (Paris). 2009. PMID: 19154698 Review. French.
-
Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways.Exp Lung Res. 2013 May-Jun;39(4-5):207-16. doi: 10.3109/01902148.2013.791733. Epub 2013 May 3. Exp Lung Res. 2013. PMID: 23638644 Review.
Cited by
-
Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease.Dev Growth Differ. 2020 Jan;62(1):67-79. doi: 10.1111/dgd.12628. Epub 2019 Oct 15. Dev Growth Differ. 2020. PMID: 31613406 Free PMC article. Review.
-
Repair and Remodeling of airway epithelium after injury in Chronic Obstructive Pulmonary Disease.Curr Respir Care Rep. 2013 Sep 1;2(3):10.1007/s13665-013-0052-2. doi: 10.1007/s13665-013-0052-2. Curr Respir Care Rep. 2013. PMID: 24187653 Free PMC article.
-
Loss of Hoxa5 function affects Hox gene expression in different biological contexts.Sci Rep. 2024 Dec 28;14(1):30903. doi: 10.1038/s41598-024-81867-0. Sci Rep. 2024. PMID: 39730789 Free PMC article.
-
Role of MAML1 and MEIS1 in Esophageal Squamous Cell Carcinoma Depth of Invasion.Pathol Oncol Res. 2018 Apr;24(2):245-250. doi: 10.1007/s12253-017-0243-1. Epub 2017 May 1. Pathol Oncol Res. 2018. PMID: 28462489
-
Furin as a therapeutic target in cystic fibrosis airways disease.Eur Respir Rev. 2023 May 3;32(168):220256. doi: 10.1183/16000617.0256-2022. Print 2023 Jun 30. Eur Respir Rev. 2023. PMID: 37137509 Free PMC article. Review.
References
-
- Aubin J., Déry U., Lemieux M., Chailler P., Jeannotte L. (2002). Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129, 4075–4087. - PubMed
-
- Bogue C. W., Gross I., Vasavada H., Dynia D. W., Wilson C. M., Jacobs H. C. (1994). Identification of Hox genes in newborn lung and effects of gestational age and retinoic acid on their expression. Am. J. Physiol. 266, L448–L454. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

