Proteomic analysis of multiple primary cilia reveals a novel mode of ciliary development in mammals
- PMID: 23213475
- PMCID: PMC3507226
- DOI: 10.1242/bio.20121081
Proteomic analysis of multiple primary cilia reveals a novel mode of ciliary development in mammals
Abstract
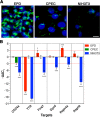
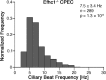
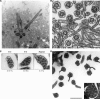
Cilia are structurally and functionally diverse organelles, whose malfunction leads to ciliopathies. While recent studies have uncovered common ciliary transport mechanisms, limited information is available on the proteome of cilia, particularly that of sensory subtypes, which could provide insight into their functional and developmental diversities. In the present study, we performed proteomic analysis of unique, multiple 9+0 cilia in choroid plexus epithelial cells (CPECs). The analysis of juvenile swine CPEC cilia identified 868 proteins. Among them, 396 were shared with the proteome of 9+0 photoreceptor cilia (outer segment), whereas only 152 were shared with the proteome of 9+2 cilia and flagella. Various signaling molecules were enriched in a CPEC-specific ciliome subset, implicating multiplicity of sensory functions. The ciliome also included molecules for ciliary motility such as Rsph9. In CPECs from juvenile swine or adult mouse, Rsph9 was localized to a subpopulation of cilia, whereas they were non-motile. Live imaging of mouse choroid plexus revealed that neonatal CPEC cilia could beat vigorously, and the motility waned and was lost within 1-2 weeks. The beating characteristics of neonatal CPEC cilia were variable and different from those of typical 9+2 cilia of ependyma, yet an Efhc1-mediated mechanism to regulate the beating frequency was shared in both types of cilia. Notably, ultrastructural analysis revealed the presence of not only 9+0 but also 9+2 and atypical ciliary subtypes in neonatal CPEC. Overall, these results identified both conserved and variable components of sensory cilia, and demonstrated a novel mode of ciliary development in mammals.
Keywords: Cilia; Development; Proteomics.
Conflict of interest statement
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

