Sca-1 is involved in the adhesion of myosphere cells to αVβ3 integrin
- PMID: 23213478
- PMCID: PMC3507234
- DOI: 10.1242/bio.20121222
Sca-1 is involved in the adhesion of myosphere cells to αVβ3 integrin
Abstract
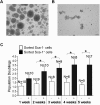
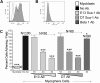
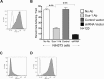
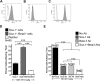
A myosphere cell is a unique type of muscle stem cell that is able to maintain its pre-myogenic state in culture over time. These cells are propagated in culture as free-floating, non-adherent spheres. We believe that the 3-dimensional adhesive cell-cell interactions involved in maintaining the sphere-like myosphere structures are also involved in maintaining their longevity in culture. We found that Sca-1, which is highly expressed by myosphere cells, plays a role in the growth and the formation of the myospheres. In comparing adhesion molecules expressed by 3-dimensionally grown myosphere cells to those expressed by 2-dimensionally grown primary myoblasts, we found that there was a distinct difference in the expression of β3 integrin. Upon further investigation we discovered that there is an adhesive interaction between Sca-1(+) cells and αVβ3 integrin. Here we show that Sca-1(+) cells (myosphere cells and NIH3T3 cells) adhere to αVβ3 integrin and that Sca-1(-) cells (primary myoblasts) do not adhere. The interaction between Sca-1 and αVβ3 integrin was confirmed using antibody blocking, shRNA knockdown of Sca-1 in Sca-1(+) cells, and by expressing Sca-1 cDNA in Sca-1(-) cells, which demonstrated that the level of adhesion of these cells to αVβ3 integrin was dependent on the presence of Sca-1. Additionally, we found that the co-expression of Sca-1 and β3 resulted in significantly greater adhesion of Sca-1(+) cells to αVβ3 integrin. In conclusion, our data indicate that Sca-1 is involved in maintaining the 3-dimensional myosphere cell-cell contacts and that Sca-1 is involved in the binding of cells to αVβ3 integrin.
Keywords: Muscle stem cells; Myosphere; Sca-1; αVβ3 integrin.
Conflict of interest statement
Figures



References
-
- Blaschuk K. L., Guérin C., Holland P. C. (1997). Myoblast αvβ3 integrin levels are controlled by transcriptional regulation of expression of the β3 subunit and down-regulation of β3 subunit expression is required for skeletal muscle cell differentiation. Dev. Biol. 184, 266–277 10.1006/dbio.1997.8527 - DOI - PubMed
-
- Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. (1996). Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85, 683–693 10.1016/S0092-8674(00)81235-0 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

