Defects in GPI biosynthesis perturb Cripto signaling during forebrain development in two new mouse models of holoprosencephaly
- PMID: 23213481
- PMCID: PMC3507239
- DOI: 10.1242/bio.20121982
Defects in GPI biosynthesis perturb Cripto signaling during forebrain development in two new mouse models of holoprosencephaly
Abstract
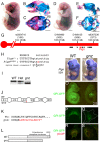
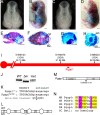
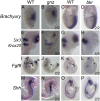
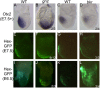
Holoprosencephaly is the most common forebrain defect in humans. We describe two novel mouse mutants that display a holoprosencephaly-like phenotype. Both mutations disrupt genes in the glycerophosphatidyl inositol (GPI) biosynthesis pathway: gonzo disrupts Pign and beaker disrupts Pgap1. GPI anchors normally target and anchor a diverse group of proteins to lipid raft domains. Mechanistically we show that GPI anchored proteins are mislocalized in GPI biosynthesis mutants. Disruption of the GPI-anchored protein Cripto (mouse) and TDGF1 (human ortholog) have been shown to result in holoprosencephaly, leading to our hypothesis that Cripto is the key GPI anchored protein whose altered function results in an HPE-like phenotype. Cripto is an obligate Nodal co-factor involved in TGFβ signaling, and we show that TGFβ signaling is reduced both in vitro and in vivo. This work demonstrates the importance of the GPI anchor in normal forebrain development and suggests that GPI biosynthesis genes should be screened for association with human holoprosencephaly.
Keywords: Cripto; GPI; Holoprosencephaly (HPE); Nodal; Pgap1; Pign; TGFβ; forebrain; glycerophosphatidyl inositol.
Conflict of interest statement
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

