Radiosynthesis and evaluation of [¹¹C-carbonyl]-labeled carbamates as fatty acid amide hydrolase radiotracers for positron emission tomography
- PMID: 23214511
- PMCID: PMC3544278
- DOI: 10.1021/jm301492y
Radiosynthesis and evaluation of [¹¹C-carbonyl]-labeled carbamates as fatty acid amide hydrolase radiotracers for positron emission tomography
Abstract
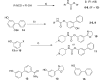
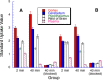
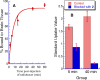
Fatty acid amide hydrolase (FAAH) plays a key role in regulating the tone of the endocannabinoid system. Radiotracers are required to image and quantify FAAH activity in vivo. We have synthesized a series of potent FAAH inhibitors encompassing two classes of N-alkyl-O-arylcarbamates and radiolabeled eight of them with carbon-11. The [¹¹C-carbonyl]-radiotracers were evaluated in vitro and ex vivo in rats as potential FAAH imaging agents for positron emission tomography (PET). Both sets of [¹¹C]O-arylcarbamates showed good to excellent brain penetration and an appropriate regional distribution. Pretreatments with a FAAH inhibitor demonstrated that 80-95% of brain uptake of radioactivity constituted binding of the radiotracers to FAAH. Brain extraction measurements showed that binding to FAAH was irreversible and kinetically different for the two classes of carbamates. These promising results are discussed in terms of the requirements of a suitable radiotracer for the in vivo imaging of FAAH using PET.
Figures

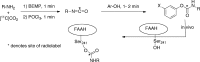
References
-
- Cravatt B. F.; Giang D. K.; Mayfield S. P.; Boger D. L.; Lerner R. A.; Gilula N. B. Molecular characterization of an enzyme that degrades neuromodulatory fatty acid amides. Nature 1996, 384, 83–87. - PubMed
- Giang D. K.; Cravatt B. F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 2238–2242. - PMC - PubMed
-
- Blankman J.; Simon G.; Cravatt B. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. - PMC - PubMed
- Dinh T. P.; Carpenter D.; Leslie F. M.; Freund T. F.; Katona I.; Sensi S. L.; Kathuria S.; Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 10819–10824. - PMC - PubMed
-
- Fowler C. J. Monoacylglycerol lipase—a target for drug development?. Br. J. Pharmacol. 2012, 166, 1568–1585. - PMC - PubMed
- Chang J. W.; Niphakis M. J.; Lum K. M.; Iii A. B. C.; Wang C.; Matthews M. L.; Niessen S.; Buczynski M. W.; Parsons L. H.; Cravatt B. F. Highly Selective Inhibitors of Monoacylglycerol Lipase Bearing a Reactive Group that Is Bioisosteric with Endocannabinoid Substrates. Chem. Biol. 2012, 19, 579–588. - PMC - PubMed
- Long J.; Li W.; Booker L.; Burston J.; Kinsey S.; Schlosburg J.; Pavón F.; Serrano A.; Selley D.; Parsons L. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chem. Biol. 2008, 5, 37–44. - PMC - PubMed
-
- McKinney M.; Cravatt B. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005, 74, 411–432. - PubMed
- Sipe J. C.; Waalen J.; Gerber A.; Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int. J. Obes. 2005, 29, 755–759. - PubMed
- Fowler C. J. The cannabinoid system and its pharmacological manipulation—a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam. Clin. Pharmacol. 2006, 20, 549–562. - PubMed
- Jayamanne A.; Greenwood R.; Mitchell V. A.; Aslan S.; Piomelli D.; Vaughan C. W. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br. J. Pharmacol. 2006, 147, 281–288. - PMC - PubMed
- Pillarisetti S.; Alexander C. W.; Khanna I. Pain and beyond: fatty acid amides and fatty acid amide hydrolase inhibitors in cardiovascular and metabolic diseases. Drug Discovery Today 2009, 14, 1098–1111. - PubMed
- Schlosburg J. E.; Kinsey S. G.; Lichtman A. H. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009, 11, 39–44. - PMC - PubMed
- Piomelli D.; Tarzia G.; Duranti A.; Tontini A.; Mor M.; Compton T. R.; Dasse O.; Monaghan E. P.; Parrott J. A.; Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006, 12, 21–38. - PMC - PubMed
- Tarzia G.; Duranti A.; Gatti G.; Piersanti G.; Tontini A.; Rivara S.; Lodola A.; Plazzi P. V.; Mor M.; Kathuria S.; Piomelli D. Synthesis and structure–activity relationships of FAAH inhibitors: cyclohexylcarbamic acid biphenyl esters with chemical modulation at the proximal phenyl ring. ChemMedChem 2006, 1, 130–139. - PubMed
- Seierstad M.; Breitenbucher J. G. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J. Med. Chem. 2008, 51, 7327–7343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

