Theoretical investigations and density functional theory based quantitative structure-activity relationships model for novel cytotoxic platinum(IV) complexes
- PMID: 23214999
- PMCID: PMC3557934
- DOI: 10.1021/jm3016427
Theoretical investigations and density functional theory based quantitative structure-activity relationships model for novel cytotoxic platinum(IV) complexes
Abstract
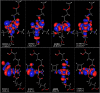
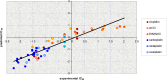
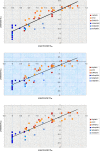
Octahedral platinum(IV) complexes are promising candidates in the fight against cancer. In order to rationalize the further development of this class of compounds, detailed studies on their mechanisms of action, toxicity, and resistance must be provided and structure-activity relationships must be drawn. Herein, we report on theoretical and QSAR investigations of a series of 53 novel bis-, tris-, and tetrakis(carboxylato)platinum(IV) complexes, synthesized and tested for cytotoxicity in our laboratories. The hybrid DFT functional wb97x was used for optimization of the structure geometry and calculation of the descriptors. Reliable and robust QSAR models with good explanatory and predictive properties were obtained for both the cisplatin sensitive cell line CH1 and the intrinsically cisplatin resistant cell line SW480, with a set of four descriptors.
Figures








References
-
- Wheate N. J.; Walker S.; Craig G. E.; Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. - PubMed
-
- Tanaka K.; Kunimatsu T.; Shimakura J.; Hanada M.. Development of Miriplatin, a Novel Antitumor Platinum for Hepatocellular Carcinoma; R&D Report, Sumitomo Kagaku, Vol. 2011-I; Dainippon Sumitomo Pharma Co., Ltd.: Osaka, Japan, 2011
-
- Yang X. Q.; Song Q. H.; Yu J. J.. Cellular and Clinical Studies of Dicycloplatin, a New Platinum Compound Approved by Chinese FDA for Cancer Chemotherapy. Presented at the 11th International Symposium on Platiumn Coordination Compounds in Cancer Chemotherapy (ISPCC in Cancer Chemotherapy), Verona, Italy, Oct 11–14, 2012.
-
- Galanski M. Recent developments in the field of anticancer platinum complexes. Recent Pat. Anti-Cancer Drug Discovery 2006, 1, 285–295. - PubMed
-
- Cleare M. J.; Hoeschele J. D. Studies on the antitumor activity of group VIII transition metal complexes. Bioinorg. Chem. 1973, 2, 187–210.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

