Identification, synthesis, and biological evaluation of metabolites of the experimental cancer treatment drugs indotecan (LMP400) and indimitecan (LMP776) and investigation of isomerically hydroxylated indenoisoquinoline analogues as topoisomerase I poisons
- PMID: 23215354
- PMCID: PMC3542640
- DOI: 10.1021/jm300519w
Identification, synthesis, and biological evaluation of metabolites of the experimental cancer treatment drugs indotecan (LMP400) and indimitecan (LMP776) and investigation of isomerically hydroxylated indenoisoquinoline analogues as topoisomerase I poisons
Abstract
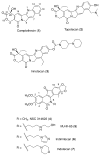
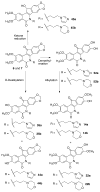
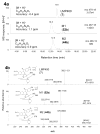
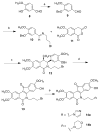
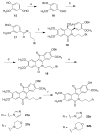
Hydroxylated analogues of the anticancer topoisomerase I (Top1) inhibitors indotecan (LMP400) and indimitecan (LMP776) have been prepared because (1) a variety of potent Top1 poisons are known that contain strategically placed hydroxyl groups, which provides a clear rationale for incorporating them in the present case, and (2) the hydroxylated compounds could conceivably serve as synthetic standards for the identification of metabolites. Indeed, incubating LMP400 and LMP776 with human liver microsomes resulted in two major metabolites of each drug, which had HPLC retention times and mass fragmentation patterns identical to those of the synthetic standards. The hydroxylated indotecan and indimitecan metabolites and analogues were tested as Top1 poisons and for antiproliferative activity in a variety of human cancer cell cultures and in general were found to be very potent. Differences in activity resulting from the placement of the hydroxyl group are explained by molecular modeling analyses.
Conflict of interest statement
Notes: The authors declare no competing financial interests.
Figures



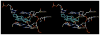






References
-
- Pommier E. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat Rev Cancer. 2006;6:789–802. - PubMed
-
- Stewart L, Redinbo MR, Qiu X, Hol WGJ, Champoux JJ. A Model for the Mechanism of Human Topoisomerase I. Science. 1998;279:1534–1541. - PubMed
-
- Wang JC. Cellular Roles of DNA Topoisomerases: A Molecular Perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

