Protein phosphatase 1 coordinates CFTR-dependent airway epithelial HCO3- secretion by reciprocal regulation of apical and basolateral membrane Cl(-)-HCO3- exchangers
- PMID: 23215877
- PMCID: PMC3623064
- DOI: 10.1111/bph.12085
Protein phosphatase 1 coordinates CFTR-dependent airway epithelial HCO3- secretion by reciprocal regulation of apical and basolateral membrane Cl(-)-HCO3- exchangers
Abstract
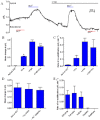
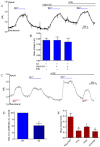
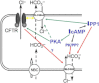
Background and purpose: Our recent studies on human airway serous-like Calu-3 cells showed that cAMP agonists stimulated a HCO3(-) rich secretion containing up to 80 mM HCO3(-). This alkaline secretion relied on a coordinated switch in the activity of distinct Cl(-)-HCO3(-) anion exchangers (AE) located at different regions of the cell. At the apical membrane, cAMP agonists activated the electroneutral AE pendrin (SLC26A4), together with cystic fibrosis transmembrane conductance regulator (CFTR), while at the basolateral membrane the agonists inhibited AE2 (SLC4A2). However, the underlying mechanism(s) that orchestrates this cAMP-dependent switch in AE activity has not been elucidated.
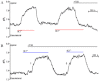
Experimental approach: Apical and basolateral Cl(-)-HCO3(-) exchange was assessed by measuring Cl(-)-dependent changes in intracellular pH (pH(i)).
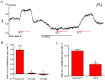
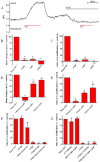
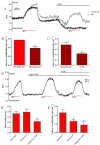
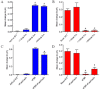
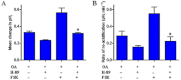
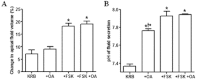
Key results: We show that protein phosphatase 1 (PP1), together with CFTR, play central roles in this reciprocal regulation of AE activity. Activation of pendrin by cAMP agonists, but not inhibition of the basolateral exchanger, was protein kinase A-dependent. Knocking down CFTR expression, or blocking its activity with GlyH-101, led to incomplete inhibition of the basolateral AE by cAMP, supporting a role for CFTR in this process. Addition of the PP1/2A inhibitor, okadaic acid, but not the PP2A specific inhibitor fostreicin, mimicked the effect of cAMP stimulation. Furthermore, okadaic acid-treated Calu-3 monolayers produced a more alkaline fluid than untreated cells, which was comparable with that produced by cAMP stimulation.
Conclusions and implications: These results identify PP1 as a novel regulator of AE activity which, in concert with CFTR, coordinates events at both apical and basolateral membranes, crucial for efficient HCO3(-) secretion from Calu-3 cells.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
References
-
- Al-Bazzaz FJ, Hafez N, Tyagi S, Gailey CA, Toofanfard M, Alrefai WA, et al. Detection of Cl-HCO3- and Na+-H+ exchangers in human airways epithelium. JOP. 2001;2:285–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

